Causes and attributable fraction of death from ARDS in inflammatory phenotypes of sepsis
- PMID: 38745253
- PMCID: PMC11092165
- DOI: 10.1186/s13054-024-04943-x
Causes and attributable fraction of death from ARDS in inflammatory phenotypes of sepsis
Abstract
Background: Hypoinflammatory and hyperinflammatory phenotypes have been identified in both Acute Respiratory Distress Syndrome (ARDS) and sepsis. Attributable mortality of ARDS in each phenotype of sepsis is yet to be determined. We aimed to estimate the population attributable fraction of death from ARDS (PAFARDS) in hypoinflammatory and hyperinflammatory sepsis, and to determine the primary cause of death within each phenotype.
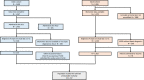
Methods: We studied 1737 patients with sepsis from two prospective cohorts. Patients were previously assigned to the hyperinflammatory or hypoinflammatory phenotype using latent class analysis. The PAFARDS in patients with sepsis was estimated separately in the hypo and hyperinflammatory phenotypes. Organ dysfunction, severe comorbidities, and withdrawal of life support were abstracted from the medical record in a subset of patients from the EARLI cohort who died (n = 130/179). Primary cause of death was defined as the organ system that most directly contributed to death or withdrawal of life support.
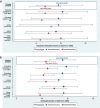
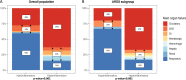
Results: The PAFARDS was 19% (95%CI 10,28%) in hypoinflammatory sepsis and, 14% (95%CI 6,20%) in hyperinflammatory sepsis. Cause of death differed between the two phenotypes (p < 0.001). Respiratory failure was the most common cause of death in hypoinflammatory sepsis, whereas circulatory shock was the most common cause in hyperinflammatory sepsis. Death with severe underlying comorbidities was more frequent in hypoinflammatory sepsis (81% vs. 67%, p = 0.004).
Conclusions: The PAFARDS is modest in both phenotypes whereas primary cause of death among patients with sepsis differed substantially by phenotype. This study identifies challenges in powering future clinical trials to detect changes in mortality outcomes among patients with sepsis and ARDS.
Keywords: Acute lung injury; Cause of death; Mortality; Phenotype; Respiratory distress syndrome; Sepsis.
© 2024. The Author(s).
Conflict of interest statement
BE received Grants for the present work from la Société de Réanimation de la langue Française, la Fondation Monohan, L’institut Servier and L’Association Limousine d’Aide aux Insuffisants Respiratoires. VEK declares a grant from the National Heart Lung and Blood Institute to his institution for the present manuscript; grants from the American Thoracic Society and Parker B. Francis Fellowship outside the present work; being part of DSMB of MODE Trial. CSC declares a grant from NIH to her institution for the present manuscript; grants from Roche Genentech, Quantum Leap Healthcare Collaborative and NIH outside the present work; consulting fees from Vasomune, Gen1e Life Science, NGM Bio, Cellenkos, Calcimedica, Arrowhead; being a co-recipient of a patent; being a council member of the International Sepsis Forum. CMH declares a grant from NIH for the present manuscript; support from DOD, NIH-NIAID and FDA to her institution outside the present work; consulting fees from Spring Discovery; being a DSMB member for regARDS Trial. KD declares being part of the University of Vermont DSBB. KDL declares grants from NIH and Quantum Leap Healthecare collaborative; consulting fees from AM Pharma, Biomerieux, Seastar Biomedical, UpToDate, Baxter; Honoraria and support from the American Society of Nephrology; and being part of advisory Boards for BOA Medical and Novartis; being an Associate Journal Editor for the American Thoracic Society. LBW declares a grant from NIH for the present manuscript; research contracts with Department of Defense, Genentech, Boehringer Ingelheim and CSL Behring; consulting fees from Arrowhead, Akebia, Santhera, Global Blood Therapeutics and Boehringer Ingelheim; being part of DSMB for CHILL Trial and SIGNET Trial; stock in Virtuoso Surgical. MAM declares grants paid to his institution outside the present work from NHLBI, NIAID, Department of Defense, Calif Institute if Regeneration, Roche Genentec and Quantum Health; consulting fees from Citius Pharmaceuticals, Gen1LifeScience, Gilead Pharmaceuticals, Novartis, Johnson and Johnson and Pilant Therapeutics. PS declares a Grant from NIGMS/NIH for the present manuscript and consulting fees from AstraZeneca and Prenosis Inc. AG, AZ, LN, NW, KNK, ES and AW declare no conflict of interests.
Figures
References
-
- Sinha P, He J, Delucchi K, Zhuo H, Abbott J, Jones C, et al. Latent class analysis-derived hypoinflammatory and hyperinflammatory phenotypes are generalisable to sepsis patients requiring intensive care. In: B95 ARDS WHATS LATEST Gt. American Thoracic Society; 2022. p. A3431–A3431.

