Granzyme B-activated IL18 potentiates αβ and γδ CAR T cell immunotherapy in a tumor-dependent manner
- PMID: 38745414
- PMCID: PMC11286818
- DOI: 10.1016/j.ymthe.2024.05.013
Granzyme B-activated IL18 potentiates αβ and γδ CAR T cell immunotherapy in a tumor-dependent manner
Abstract
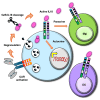
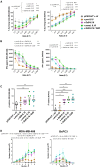
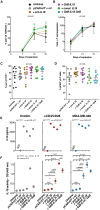
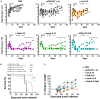
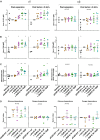
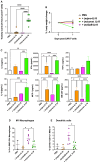
Interleukin (IL)18 is a potent pro-inflammatory cytokine that is activated upon caspase 1 cleavage of the latent precursor, pro-IL18. Therapeutic T cell armoring with IL18 promotes autocrine stimulation and positive modulation of the tumor microenvironment (TME). However, existing strategies are imperfect since they involve constitutive/poorly regulated activity or fail to modify the TME. Here, we have substituted the caspase 1 cleavage site within pro-IL18 with that preferred by granzyme B, yielding GzB-IL18. We demonstrate that GzB-IL18 is constitutively released but remains functionally latent unless chimeric antigen receptor (CAR) T cells are activated, owing to concomitant granzyme B release. Armoring with GzB-IL18 enhances cytolytic activity, proliferation, interferon (IFN)-γ release, and anti-tumor efficacy by a similar magnitude to constitutively active IL18. We also demonstrate that GzB-IL18 provides a highly effective armoring strategy for γδ CAR T cells, leading to enhanced metabolic fitness and significant potentiation of therapeutic activity. Finally, we show that constitutively active IL18 can unmask CAR T cell-mediated cytokine release syndrome in immunocompetent mice. By contrast, GzB-IL18 promotes anti-tumor activity and myeloid cell re-programming without inducing such toxicity. Using this stringent system, we have tightly coupled the biological activity of IL18 to the activation state of the host CAR T cell, favoring safer clinical implementation of this technology.
Keywords: CAR T-cell; Chimeric antigen receptor; IL18; cancer; granzyme B; immunotherapy; αβ T cell; γδ T cell.
Copyright © 2024 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests J.M. is founding scientist, shareholder, consultant, and Chief Scientific Officer of Leucid Bio. C.M.H., R.M., and D.M.D. are currently employees of Leucid Bio. J.M. and C.M.H. are named inventors on patent filings that pertain to granzyme B-regulated cytokines.
Figures



References
-
- Chmielewski M., Abken H. TRUCKs, the fourth generation CAR T cells: Current developments and clinical translation. Adv. Cell Gene Ther. 2020;3 doi: 10.1002/acg2.84. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

