Induction of diabetes by Tacrolimus in a phenotypic model of obesity and metabolic syndrome
- PMID: 38745946
- PMCID: PMC11092379
- DOI: 10.3389/fendo.2024.1388361
Induction of diabetes by Tacrolimus in a phenotypic model of obesity and metabolic syndrome
Abstract
Introduction: The pathogenesis of Post-Transplant Diabetes Mellitus (PTDM) is complex and multifactorial and it resembles that of Type-2 Diabetes Mellitus (T2DM). One risk factor specific to PTDM differentiates both entities: the use of immunosuppressive therapy. Specifically, Tacrolimus interacts with obesity and insulin resistance (IR) in accelerating the onset of PTDM. In a genotypic model of IR, the obese Zucker rats, Tacrolimus is highly diabetogenic by promoting the same changes in beta-cell already modified by IR. Nevertheless, genotypic animal models have their limitations and may not resemble the real pathophysiology of diabetes. In this study, we have evaluated the interaction between beta-cell damage and Tacrolimus in a non-genotypic animal model of obesity and metabolic syndrome.
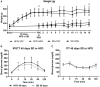
Methods: Sprague Dawley rats were fed a high-fat enriched diet during 45 days to induce obesity and metabolic dysregulation. On top of this established obesity, the administration of Tacrolimus (1mg/kg/day) during 15 days induced severe hyperglycaemia and changes in morphological and structural characteristics of the pancreas.
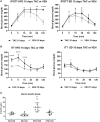
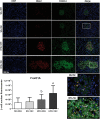
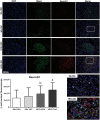
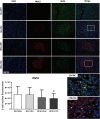
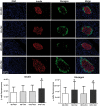
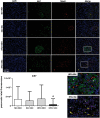
Results: Obese animals administered with Tacrolimus showed increased size of islets of Langerhans and reduced beta-cell proliferation without changes in apoptosis. There were also changes in beta-cell nuclear factors such as a decrease in nuclear expression of MafA and a nuclear overexpression of FoxO1A, PDX-1 and NeuroD1. These animals also showed increased levels of pancreatic insulin and glucagon.
Discussion: This model could be evidence of the relationship between the T2DM and PTDM physiopathology and, eventually, the model may be instrumental to study the pathogenesis of T2DM.
Keywords: Tacrolimus; animal model; metabolic syndrome; obesity; post-transplant diabetes; type 2 diabetes.
Copyright © 2024 Teixidó-Trujillo, Porrini, Menéndez-Quintanal, Torres-Ramírez, Fumero and Rodríguez-Rodríguez.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Rodriguez-Rodriguez AE, Triñanes J, Velazquez-Garcia S, Porrini E, Vega Prieto MJ, Diez Fuentes ML, et al. . The higher diabetogenic risk of tacrolimus depends on pre-existing insulin resistance. A study in obese and lean zucker rats. Am J Transplant. (2013) 13:1665–75. doi: 10.1111/ajt.12236 - DOI - PubMed
-
- Pham PT, Sarkar M, Pham PM, Pham PC. Diabetes mellitus after solid organ transplantation. (2000) South Dartmouth (MA): MDText.com. Available at: https://www.ncbi.nlm.nih.gov/books/NBK378977/.
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

