This is a preprint.
Cortical circuit principles predict patterns of trauma induced tauopathy in humans
- PMID: 38746103
- PMCID: PMC11092596
- DOI: 10.1101/2024.05.02.592271
Cortical circuit principles predict patterns of trauma induced tauopathy in humans
Update in
-
Cortical circuit principles predict patterns of trauma induced tauopathy in humans.Cereb Cortex. 2025 Aug 1;35(8):bhaf209. doi: 10.1093/cercor/bhaf209. Cereb Cortex. 2025. PMID: 40817910
Abstract
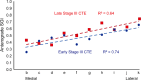
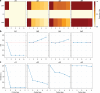
Connections in the cortex of diverse mammalian species are predicted reliably by the Structural Model for direction of pathways and signal processing (reviewed in 1,2). The model is rooted in the universal principle of cortical systematic variation in laminar structure and has been supported widely for connection patterns in animals but has not yet been tested for humans. Here, in postmortem brains of individuals neuropathologically diagnosed with chronic traumatic encephalopathy (CTE) we studied whether the hyperphosphorylated tau (p-tau) pathology parallels connection sequence in time by circuit mechanisms. CTE is a progressive p-tau pathology that begins focally in perivascular sites in sulcal depths of the neocortex (stages I-II) and later involves the medial temporal lobe (MTL) in stages III-IV. We provide novel quantitative evidence that the p-tau pathology in MTL A28 and nearby sites in CTE stage III closely follows the graded laminar patterns seen in homologous cortico-cortical connections in non-human primates. The Structural Model successfully predicted the laminar distribution of the p-tau neurofibrillary tangles and neurites and their density, based on the relative laminar (dis)similarity between the cortical origin (seed) and each connection site. The findings were validated for generalizability by a computational progression model. By contrast, the early focal perivascular pathology in the sulcal depths followed local columnar connectivity rules. These findings support the general applicability of a theoretical model to unravel the direction and progression of p-tau pathology in human neurodegeneration via a cortico-cortical mechanism. Cortical pathways converging on medial MTL help explain the progressive spread of p-tau pathology from focal cortical sites in early CTE to widespread lateral MTL areas and beyond in later disease stages.
Conflict of interest statement
Conflict of interest statement Dr. McKee is a member of the Mackey-White Committee of the National Football League Players Association. The remaining authors declare no competing interests.
Figures




Similar articles
-
Cortical circuit principles predict patterns of trauma induced tauopathy in humans.Cereb Cortex. 2025 Aug 1;35(8):bhaf209. doi: 10.1093/cercor/bhaf209. Cereb Cortex. 2025. PMID: 40817910
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
Short-Term Memory Impairment.2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31424720 Free Books & Documents.
-
Neuropathology in chronic traumatic encephalopathy: a systematic review of comparative post-mortem histology literature.Acta Neuropathol Commun. 2022 Aug 6;10(1):108. doi: 10.1186/s40478-022-01413-9. Acta Neuropathol Commun. 2022. PMID: 35933388 Free PMC article.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
References
-
- Barbas H. General cortical and special prefrontal connections: Principles from structure to function. Annu Rev Neurosci 38, 269–289 (2015). - PubMed
-
- Pandya D., Seltzer B., Petrides M. & Cipolloni P. B. Cerebral Cortex: Architecture, Connections, and the Dual Origin Concept. (Oxford University Press, 2015).
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
