This is a preprint.
MmpL3, Wag31 and PlrA are involved in coordinating polar growth with peptidoglycan metabolism and nutrient availability
- PMID: 38746181
- PMCID: PMC11092516
- DOI: 10.1101/2024.04.29.591792
MmpL3, Wag31 and PlrA are involved in coordinating polar growth with peptidoglycan metabolism and nutrient availability
Update in
-
MmpL3, Wag31, and PlrA are involved in coordinating polar growth with peptidoglycan metabolism and nutrient availability.J Bacteriol. 2024 Oct 24;206(10):e0020424. doi: 10.1128/jb.00204-24. Epub 2024 Sep 25. J Bacteriol. 2024. PMID: 39320104 Free PMC article.
Abstract
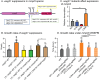
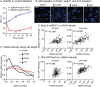
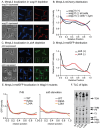
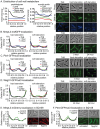
Cell growth in mycobacteria involves cell wall expansion that is restricted to the cell poles. The DivIVA homolog Wag31 is required for this process, but the molecular mechanism and protein partners of Wag31 have not been described. In this study of Mycobacterium smegmatis, we identify a connection between wag31 and trehalose monomycolate (TMM) transporter mmpl3 in a suppressor screen, and show that Wag31 and polar regulator PlrA are required for MmpL3's polar localization. In addition, the localization of PlrA and MmpL3 are responsive to nutrient and energy deprivation and inhibition of peptidoglycan metabolism. We show that inhibition of MmpL3 causes delocalized cell wall metabolism, but does not delocalize MmpL3 itself. We found that cells with an MmpL3 C-terminal truncation, which is defective for localization, have only minor defects in polar growth, but are impaired in their ability to downregulate cell wall metabolism under stress. Our work suggests that, in addition to its established function in TMM transport, MmpL3 has a second function in regulating global cell wall metabolism in response to stress. Our data are consistent with a model in which the presence of TMMs in the periplasm stimulates polar elongation, and in which the connection between Wag31, PlrA and the C-terminus of MmpL3 is involved in detecting and responding to stress in order to coordinate synthesis of the different layers of the mycobacterial cell wall in changing conditions.
Keywords: DivIVA; MmpL3; Murj; Mycobacterium; Mycolic acid; PlrA; TMMs; peptidoglycan; polar growth regulation; stress condition.
Figures
References
-
- Minnikin DE. 1991. Chemical principles in the organization of lipid components in the mycobacterial cell envelope. Research in Microbiology 142:423–427. - PubMed
-
- Thanky NR, Young DB, Robertson BD. 2007. Unusual features of the cell cycle in mycobacteria: Polar-restricted growth and the snapping-model of cell division. Tuberculosis 87:231–236. - PubMed
-
- Daffé M, Draper P. 1997. The Envelope Layers of Mycobacteria with Reference to their Pathogenicity, p. 131–203. In Advances in Microbial Physiology. Elsevier. - PubMed
-
- McNeil M, Wallner SJ, Hunter SW, Brennan PJ. 1987. Demonstration that the galactosyl and arabinosyl residues in the cell-wall arabinogalactan of Mycobacterium leprae and Myobacterium tuberculosis are furanoid. Carbohydrate Research 166:299–308. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
