This is a preprint.
Transcription of HIV-1 at sites of intact latent provirus integration
- PMID: 38746186
- PMCID: PMC11092494
- DOI: 10.1101/2024.04.26.591331
Transcription of HIV-1 at sites of intact latent provirus integration
Update in
-
Transcription of HIV-1 at sites of intact latent provirus integration.J Exp Med. 2024 Sep 2;221(9):e20240391. doi: 10.1084/jem.20240391. Epub 2024 Aug 14. J Exp Med. 2024. PMID: 39141127 Free PMC article.
Abstract
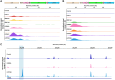
HIV-1 anti-retroviral therapy is highly effective but fails to eliminate a reservoir of latent proviruses leading to a requirement for life-long treatment. How the site of integration of authentic intact latent proviruses might impact their own or neighboring gene expression or reservoir dynamics is poorly understood. Here we report on proviral and neighboring gene transcription at sites of intact latent HIV-1 integration in cultured T cells obtained directly from people living with HIV, as well as engineered primary T cells and cell lines. Proviral gene expression was correlated to the level of endogenous gene expression under resting but not activated conditions. Notably, latent proviral promoters were 10010,000X less active than in productively infected cells and had little or no measurable impact on neighboring gene expression under resting or activated conditions. Thus, the site of integration has a dominant effect on the transcriptional activity of intact HIV-1 proviruses in the latent reservoir thereby influencing cytopathic effects and proviral immune evasion.
Conflict of interest statement
The authors declare no competing financial interests.
Figures




References
-
- Antar A.A.R., Jenike K.M., Jang S., Rigau D.N., Reeves D.B., Hoh R., Krone M.R., Keruly J.C., Moore R.D., Schiffer J.T., Nonyane B.A.S., Hecht F.M., Deeks S.G., Siliciano J.D., Ho Y.-C., and Siliciano R.F.. 2020. Longitudinal study reveals HIV-1–infected CD4+ T cell dynamics during long-term antiretroviral therapy. Journal of Clinical Investigation 130:3543–3559. - PMC - PubMed
-
- Bachmann N., von Siebenthal C., Vongrad V., Turk T., Neumann K., Beerenwinkel N., Bogojeska J., Fellay J., Roth V., Kok Y.L., Thorball C.W., Borghesi A., Parbhoo S., Wieser M., Boni J., Perreau M., Klimkait T., Yerly S., Battegay M., Rauch A., Hoffmann M., Bernasconi E., Cavassini M., Kouyos R.D., Gunthard H.F., Metzner K.J., and Swiss H.I.V.C.S.. 2019. Determinants of HIV-1 reservoir size and long-term dynamics during suppressive ART. Nat Commun 10:3193. - PMC - PubMed
-
- Bui J.K., Sobolewski M.D., Keele B.F., Spindler J., Musick A., Wiegand A., Luke B.T., Shao W., Hughes S.H., Coffin J.M., Kearney M.F., and Mellors J.W.. 2017. Proviruses with identical sequences comprise a large fraction of the replication-competent HIV reservoir. PLOS Pathogens 13:e1006283. - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
