This is a preprint.
STRUCTURE-FUNCTION RELATIONSHIPS OF MUCOCILIARY CLEARANCE IN HUMAN AIRWAYS
- PMID: 38746209
- PMCID: PMC11092836
- DOI: 10.21203/rs.3.rs-4164522/v1
STRUCTURE-FUNCTION RELATIONSHIPS OF MUCOCILIARY CLEARANCE IN HUMAN AIRWAYS
Update in
-
Structure and function relationships of mucociliary clearance in human and rat airways.Nat Commun. 2025 Mar 12;16(1):2446. doi: 10.1038/s41467-025-57667-z. Nat Commun. 2025. PMID: 40069153 Free PMC article.
Abstract
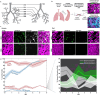
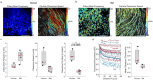
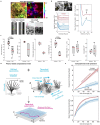
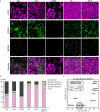
Our study focuses on the intricate connection between tissue-level organization and ciliated organ function in humans, particularly in understanding the morphological organization of airways and their role in mucociliary clearance. Mucociliary clearance is a key mechanical defense mechanism of human airways, and clearance failure is associated with many respiratory diseases, including chronic obstructive pulmonary disease (COPD) and asthma. While single-cell transcriptomics have unveiled the cellular complexity of the human airway epithelium, our understanding of the mechanics that link epithelial structure to clearance function mainly stem from animal models. This reliance on animal data limits crucial insights into human airway barrier function and hampers the human-relevant in vitro modeling of airway diseases. This study, for the first time, maps the distribution of ciliated and secretory cell types along the airway tree in both rats and humans, noting species-specific differences in ciliary function and elucidates structural parameters of airway epithelia that predict clearance function in both native and in vitro tissues alike. By uncovering how tissue organization influences ciliary function, we can better understand disruptions in mucociliary clearance, which could have implications for various ciliated organs beyond the airways.
Conflict of interest statement
COMPETING INTERESTS DECLARATIONS The authors declare no competing interests.
Figures


References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

