This is a preprint.
Growth of Staphylococcus aureus in the presence of oleic acid shifts the glycolipid fatty acid profile and increases resistance to antimicrobial peptides
- PMID: 38746422
- PMCID: PMC11092785
- DOI: 10.1101/2024.05.03.592415
Growth of Staphylococcus aureus in the presence of oleic acid shifts the glycolipid fatty acid profile and increases resistance to antimicrobial peptides
Update in
-
Growth of Staphylococcus aureus in the presence of oleic acid shifts the glycolipid fatty acid profile and increases resistance to antimicrobial peptides.Biochim Biophys Acta Biomembr. 2025 Jan;1867(1):184395. doi: 10.1016/j.bbamem.2024.184395. Epub 2024 Nov 3. Biochim Biophys Acta Biomembr. 2025. PMID: 39500386
Abstract
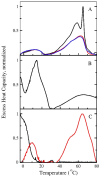
Staphylococcus aureus readily adapts to various environments and quickly develops antibiotic resistance, which has led to an increase in multidrug-resistant infections. Hence, S. aureus presents a significant global health issue and its adaptations to the host environment are crucial for understanding pathogenesis and antibiotic susceptibility. When S. aureus is grown conventionally, its membrane lipids contain a mix of branched-chain and straight-chain saturated fatty acids. However, when unsaturated fatty acids are present in the growth medium, they become a major part of the total fatty acid composition. This study explores the biophysical effects of incorporating straight-chain unsaturated fatty acids into S. aureus membrane lipids. Membrane preparations from cultures supplemented with oleic acid showed more complex differential scanning calorimetry scans than those grown in tryptic soy broth alone. When grown in the presence of oleic acid, the cultures exhibited a transition significantly above the growth temperature, attributed to the presence of glycolipids with long-chain fatty acids causing acyl chain packing frustration within the bilayer. Functional aspects of the membrane were assessed by studying the kinetics of dye release from unilamellar vesicles induced by the antimicrobial peptide mastoparan X. Dye release was slower from liposomes prepared from cells grown in oleic acid-supplemented cultures, suggesting that changes in membrane lipid composition and biophysics protect the cell membrane against peptide-induced lysis. These findings underscore the intricate relationship between the growth environment, membrane lipid composition, and the physical properties of the bacterial membrane, which should be considered when developing new strategies against S. aureus infections.
Figures




Similar articles
-
Growth of Staphylococcus aureus in the presence of oleic acid shifts the glycolipid fatty acid profile and increases resistance to antimicrobial peptides.Biochim Biophys Acta Biomembr. 2025 Jan;1867(1):184395. doi: 10.1016/j.bbamem.2024.184395. Epub 2024 Nov 3. Biochim Biophys Acta Biomembr. 2025. PMID: 39500386
-
Growth-Environment Dependent Modulation of Staphylococcus aureus Branched-Chain to Straight-Chain Fatty Acid Ratio and Incorporation of Unsaturated Fatty Acids.PLoS One. 2016 Oct 27;11(10):e0165300. doi: 10.1371/journal.pone.0165300. eCollection 2016. PLoS One. 2016. PMID: 27788193 Free PMC article.
-
Branched phospholipids render lipid vesicles more susceptible to membrane-active peptides.Biochim Biophys Acta. 2016 May;1858(5):988-94. doi: 10.1016/j.bbamem.2015.10.014. Epub 2015 Oct 26. Biochim Biophys Acta. 2016. PMID: 26514602 Free PMC article.
-
Lipidomic and Ultrastructural Characterization of the Cell Envelope of Staphylococcus aureus Grown in the Presence of Human Serum.mSphere. 2020 Jun 17;5(3):e00339-20. doi: 10.1128/mSphere.00339-20. mSphere. 2020. PMID: 32554713 Free PMC article.
-
Lipidomics of homeoviscous adaptation to low temperatures in Staphylococcus aureus utilizing exogenous straight-chain unsaturated fatty acids.J Bacteriol. 2024 Jul 25;206(7):e0018724. doi: 10.1128/jb.00187-24. Epub 2024 Jul 2. J Bacteriol. 2024. PMID: 38953643 Free PMC article.
References
-
- World Health Organization, Prioritization of pathogens to guide discovery, research and development of new antibiotics for drug-resistant bacterial infections, including tuberculosis, World Health Organization, 2017.
-
- 2021 antibacterial agents in clinical and preclinical development: an overview and analysis, (n.d.).
-
- Garber E.D., THE HOST AS A GROWTH MEDIUM, Ann. N. Y. Acad. Sci., 88 (1960) 1187–1194. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
