This is a preprint.
In vitro heterochronic parabiosis identifies pigment epithelium-derived factor as a systemic mediator of rejuvenation by young blood
- PMID: 38746475
- PMCID: PMC11092633
- DOI: 10.1101/2024.05.02.592258
In vitro heterochronic parabiosis identifies pigment epithelium-derived factor as a systemic mediator of rejuvenation by young blood
Abstract
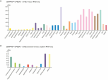
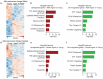
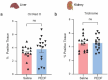
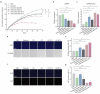
Several decades of heterochronic parabiosis (HCPB) studies have demonstrated the restorative impact of young blood, and deleterious influence of aged blood, on physiological function and homeostasis across tissues, although few of the factors responsible for these observations have been identified. Here we develop an in vitro HCPB system to identify these circulating factors, using replicative lifespan (RLS) of primary human fibroblasts as an endpoint of cellular health. We find that RLS is inversely correlated with serum donor age and sensitive to the presence or absence of specific serum components. Through in vitro HCPB, we identify the secreted protein pigment epithelium-derived factor (PEDF) as a circulating factor that extends RLS of primary human fibroblasts and declines with age in mammals. Systemic administration of PEDF to aged mice reverses age-related functional decline and pathology across several tissues, improving cognitive function and reducing hepatic fibrosis and renal lipid accumulation. Together, our data supports PEDF as a systemic mediator of the effect of young blood on organismal health and homeostasis and establishes our in vitro HCPB system as a valuable screening platform for the identification of candidate circulating factors involved in aging and rejuvenation.
Conflict of interest statement
Competing interests The authors declare no competing interests.
Figures






References
-
- Conboy I.M. et al. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature 433, 760–4 (2005). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
