Tick-borne zoonotic flaviviruses and Borrelia infections in wildlife hosts: What have field studies contributed?
- PMID: 38746540
- PMCID: PMC11091708
- DOI: 10.1016/j.onehlt.2024.100747
Tick-borne zoonotic flaviviruses and Borrelia infections in wildlife hosts: What have field studies contributed?
Abstract
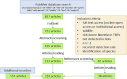
Tick-borne flaviviruses and Borrelia spp. are globally spread pathogens of zoonotic potential that are maintained by a transmission cycle at the interface between ticks and vertebrate hosts, mainly wild animals. Aside data on pathogen burden in ticks, information on the status of various hosts relative to infection is important to acquire. We reviewed how those infections have been studied in wildlife host species in the field to discuss how collected data provided relevant epidemiological information and to identify needs for further studies. The literature was screened for observational studies on pathogen or antibody detection for tick-borne Borrelia spp. and flaviviruses in wildlife host animals. Overall, Borrelia spp. were more studied (73% of case studies, representing 297 host species) than flaviviruses (27% of case studies, representing 114 host species). Studies on both Borrelia spp. and flaviviruses focused mainly on the same species, namely bank vole and yellow-necked mouse. Most studies were order-specific and cross-sectional, reporting prevalence at various locations, but with little insight into the underlying epidemiological dynamics. Host species with potential to act as reservoir hosts of these pathogens were neglected, notably birds. We highlight the necessity of collecting both demographics and infection data in wildlife studies, and to consider communities of species, to better estimate zoonotic risk potential in the One Health context.
Keywords: Borrelia; Flavivirus; Host; Reservoir; Sentinel; Tick-borne diseases; Wildlife.
© 2024 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

References
-
- Bouchard É., Sharma R., Hernández-Ortiz A., Buhler K., Al-Adhami B., Su C., Fenton H., Gouin G.G., Roth J.D., Rodrigues C.W., Pamak C., Simon A., Bachand N., Leighton P., Jenkins E. Are foxes (Vulpes spp.) good sentinel species for toxoplasma gondii in northern Canada? Parasit. Vectors. 2022;15:115. doi: 10.1186/s13071-022-05229-3. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

