Innovation of 6-sulfonamide-2 H-chromene derivatives as antidiabetic agents targeting α-amylase, α-glycosidase, and PPAR-γ inhibitors with in silico molecular docking simulation
- PMID: 38746843
- PMCID: PMC11091863
- DOI: 10.1039/d4ra02143f
Innovation of 6-sulfonamide-2 H-chromene derivatives as antidiabetic agents targeting α-amylase, α-glycosidase, and PPAR-γ inhibitors with in silico molecular docking simulation
Abstract
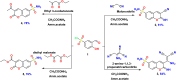
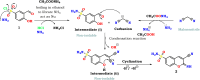
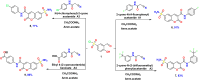
A new series of 2-imino or 2-oxo-2H-chromene-6-sulfonamide derivatives 2-9 with potential anti-diabetic activity were designed and synthesized. The new 6-sulfonamide chromenes were synthesized by reacting 3-formyl-4-hydroxybenzenesulfonyl chloride with activated methylene derivatives in the presence of ammonium acetate as a catalyst. The structure of the products was confirmed by spectroscopic analysis. All the designed derivatives 2-9 were evaluated for their activity against α-amylase and exhibited inhibitory percentage values higher than 93% at 100 μg mL-1. Additionally, the IC50 values represented a variable degree of activity with two derivatives 2 and 9 exhibiting the most promising derivative results with IC50 values of 1.76 ± 0.01 and 1.08 ± 0.02 μM, respectively, compared to Acarbose (IC50 = 0.43 ± 0.01 μM). Additionally, these derivatives showed potency against the α-glucosidase enzyme with IC50 values of 0.548 ± 0.02 and 2.44 ± 0.09 μg mL-1, compared to Acarbose (0.604 ± 0.02 μg mL-1). Moreover, the in vitro PPAR-γ transactivation assay revealed that chromene-6-sulfonamide derivatives 2 and 9 exhibited potential PPAR-γ activity with IC50 values of 3.152 ± 0.03 and 3.706 ± 0.32 μg mL-1, respectively, compared to Pioglitazone (4.884 ± 0.29 μg mL-1). This indicates that these derivatives have insulin sensitivity and glucose metabolism activity. The in silico ADMET prediction showed that these derivatives have an acceptable range of oral bioavailability, drug-likeness, and a safe toxicity profile, including being non-cytotoxic, non-mutagenic, non-immunotoxic, and non-carcinogenic. Finally, computational docking analysis demonstrated the ability of these derivatives to interact with α-amylase, α-glucosidase, and PPAR-γ enzymes, with confirmed successful placement due to good binding energy values and various interactions within the pocket.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





References
-
- Cho N. H. Shaw J. E. Karuranga S. Huang Y. da Rocha Fernandes J. D. Ohlrogge A. W. Malanda B. Diabetes Res. Clin. Pract. 2018;138:271–281. - PubMed
-
- Association A. D. Diabetes Care. 2013;37:S81–S90.
-
- Adogu P. O. U. Chineke H. N. Ewuzie M. U. Enwere O. O. Egenti N. B. J. Diabetes Mellitus. 2015;5:49.
LinkOut - more resources
Full Text Sources

