Characterization of innate lymphoid cell subsets infiltrating melanoma and epithelial ovarian tumors
- PMID: 38746870
- PMCID: PMC11093043
- DOI: 10.1080/2162402X.2024.2349347
Characterization of innate lymphoid cell subsets infiltrating melanoma and epithelial ovarian tumors
Abstract
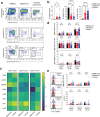
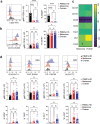
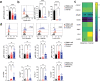
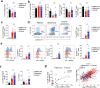
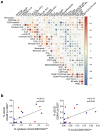
The innate lymphoid cell (ILC) family is composed of heterogeneous innate effector and helper immune cells that preferentially reside in tissues where they promote tissue homeostasis. In cancer, they have been implicated in driving both pro- and anti-tumor responses. This apparent dichotomy highlights the need to better understand differences in the ILC composition and phenotype within different tumor types that could drive seemingly opposite anti-tumor responses. Here, we characterized the frequency and phenotype of various ILC subsets in melanoma metastases and primary epithelial ovarian tumors. We observed high PD-1 expression on ILC subsets isolated from epithelial ovarian tumor samples, while ILC populations in melanoma samples express higher levels of LAG-3. In addition, we found that the frequency of cytotoxic ILCs and NKp46+ILC3 in tumors positively correlates with monocytic cells and conventional type 2 dendritic cells, revealing potentially new interconnected immune cell subsets in the tumor microenvironment. Consequently, these observations may have direct relevance to tumor microenvironment composition and how ILC subset may influence anti-tumor immunity.
Keywords: Epithelial ovarian cancer; LAG-3; NK cells; PD-1; T cells; innate lymphoid cells; melanoma; myeloid cells.
© 2024 The Author(s). Published with license by Taylor & Francis Group, LLC.
Conflict of interest statement
Dr. Pamela Ohashi is on Scientific Advisory Boards for Providence Therapeutics, Treadwell Therapeutics, Tikva Allocell and Rondo Therapeutics. Inc. Dr. Ohashi holds a sponsored research agreement with Providence Therapeutics. No potential conflict of interest was reported by the other authors.
Figures



References
-
- Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L, Burow M. et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10(9):942–949. doi:10.1038/nm1093. - DOI - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
