Synthesis and in Vitro Evaluation of CAPE Derivatives as Ferroptosis Inducers in Triple Negative Breast Cancer
- PMID: 38746881
- PMCID: PMC11089544
- DOI: 10.1021/acsmedchemlett.4c00099
Synthesis and in Vitro Evaluation of CAPE Derivatives as Ferroptosis Inducers in Triple Negative Breast Cancer
Abstract
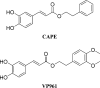
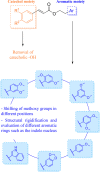
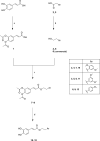
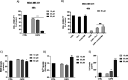
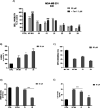
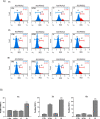
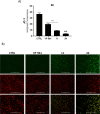
Herein, we describe the design, synthesis, and in vitro biological evaluation of HO-1 inducers endowed with cytotoxic effects mediated by ferroptosis activation. Using the natural HO-1 inducer caffeic acid phenethyl ester (CAPE) as a chemical scaffold, new derivatives were synthesized by performing modifications in the cathecol moiety and in the phenethyl ester aromatic ring. Biological assays aimed at evaluating an imbalanced activity of ferroptosis key players identified that 2-(1H-indol-3-yl)ethyl cinnamate (compound 24) possesses improved anticancer activity toward the MDA-MB 231 triple negative breast cancer cell line when compared to CAPE. Increased ROS and LOOH levels, reduced GSH levels, imbalanced mitochondrial activity, and restored cell viability after ferrostatin-1 treatment suggested a ferroptotic mechanism of action, which did not involve GPX4 inhibition. Compound 24 represents an intriguing hit compound useful for the identification of novel ferroptosis inducers.
© 2024 American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

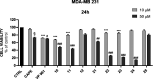

References
LinkOut - more resources
Full Text Sources
Miscellaneous

