Neoadjuvant treatment of colorectal cancer: comprehensive review
- PMID: 38747103
- PMCID: PMC11094476
- DOI: 10.1093/bjsopen/zrae038
Neoadjuvant treatment of colorectal cancer: comprehensive review
Abstract
Background: Neoadjuvant therapy has an established role in the treatment of patients with colorectal cancer. However, its role continues to evolve due to both advances in the available treatment modalities, and refinements in the indications for neoadjuvant treatment and subsequent surgery.
Methods: A narrative review of the most recent relevant literature was conducted.
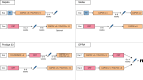
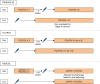
Results: Short-course radiotherapy and long-course chemoradiotherapy have an established role in improving local but not systemic disease control in patients with rectal cancer. Total neoadjuvant therapy offers advantages over short-course radiotherapy and long-course chemoradiotherapy, not only in terms of increased local response but also in reducing the risk of systemic relapses. Non-operative management is increasingly preferred to surgery in patients with rectal cancer and clinical complete responses but is still associated with some negative impacts on functional outcomes. Neoadjuvant chemotherapy may be of some benefit in patients with locally advanced colon cancer with proficient mismatch repair, although patient selection is a major challenge. Neoadjuvant immunotherapy in patients with deficient mismatch repair cancers in the colon or rectum is altering the treatment paradigm for these patients.
Conclusion: Neoadjuvant treatments for patients with colon or rectal cancers continue to evolve, increasing the complexity of decision-making for patients and clinicians alike. This review describes the current guidance and most recent developments.
© The Author(s) 2024. Published by Oxford University Press on behalf of BJS Foundation Ltd.
Figures
References
-
- Hurst PA, Prout WG, Kelly JM, Bannister JJ, Walker RT. Local recurrence after low anterior resection using the staple gun. Br J Surg 1982;69:275–276 - PubMed
-
- Lasson AL, Ekelund GR, Lindstrom CG. Recurrence risk after stapled anastomosis for rectal carcinoma. Acta Chir Scand 1984;150:85–89 - PubMed
-
- Heald RJ, Husband EM, Ryall RD. The mesorectum in rectal cancer surgery—the clue to pelvic recurrence? Br J Surg 1982;69:613–616 - PubMed
-
- Heald RJ, Ryall RD. Recurrence and survival after total mesorectal excision for rectal cancer. Lancet 1986;1:1479–1482 - PubMed
-
- Heald RJ, Moran BJ, Ryall RD, Sexton R, MacFarlane JK. Rectal cancer: the Basingstoke experience of total mesorectal excision, 1978–1997. Arch Surg 1998;133:894–899 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

