Associations among plasma, MRI, and amyloid PET biomarkers of Alzheimer's disease and related dementias and the impact of health-related comorbidities in a community-dwelling cohort
- PMID: 38747525
- PMCID: PMC11180870
- DOI: 10.1002/alz.13835
Associations among plasma, MRI, and amyloid PET biomarkers of Alzheimer's disease and related dementias and the impact of health-related comorbidities in a community-dwelling cohort
Abstract
Introduction: We evaluated associations between plasma and neuroimaging-derived biomarkers of Alzheimer's disease and related dementias and the impact of health-related comorbidities.
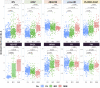
Methods: We examined plasma biomarkers (neurofilament light chain, glial fibrillary acidic protein, amyloid beta [Aβ] 42/40, phosphorylated tau 181) and neuroimaging measures of amyloid deposition (Aβ-positron emission tomography [PET]), total brain volume, white matter hyperintensity volume, diffusion-weighted fractional anisotropy, and neurite orientation dispersion and density imaging free water. Participants were adjudicated as cognitively unimpaired (CU; N = 299), mild cognitive impairment (MCI; N = 192), or dementia (DEM; N = 65). Biomarkers were compared across groups stratified by diagnosis, sex, race, and APOE ε4 carrier status. General linear models examined plasma-imaging associations before and after adjusting for demographics (age, sex, race, education), APOE ε4 status, medications, diagnosis, and other factors (estimated glomerular filtration rate [eGFR], body mass index [BMI]).
Results: Plasma biomarkers differed across diagnostic groups (DEM > MCI > CU), were altered in Aβ-PET-positive individuals, and were associated with poorer brain health and kidney function.
Discussion: eGFR and BMI did not substantially impact associations between plasma and neuroimaging biomarkers.
Highlights: Plasma biomarkers differ across diagnostic groups (DEM > MCI > CU) and are altered in Aβ-PET-positive individuals. Altered plasma biomarker levels are associated with poorer brain health and kidney function. Plasma and neuroimaging biomarker associations are largely independent of comorbidities.
Keywords: dementias; neuroimaging; plasma biomarkers.
© 2024 The Authors. Alzheimer's & Dementia published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Conflict of interest statement
The authors declare the following financial interests/personal relationships, which may be considered as potential competing interests: Marc Rudolph, Courtney Sutphen, Thomas Register, Christopher Whitlow, Kiran Solingapuram Sai, Timothy Hughes, and Kristen Russ have no conflicts of interest to disclose. James R. Bateman and Samuel N. Lockhart receive funding from the NIH and Alzheimer's Association. Dage is an inventor on patents or patent applications of Eli Lilly and Company relating to the assays, methods, reagents, and/or compositions of matter for p‐tau assays. Dage has served as a consultant or on advisory boards for Eisai, Abbvie, Genotix Biotechnologies Inc., Gates Ventures, Karuna Therapeutics, AlzPath Inc., and Cognito Therapeutics, Inc., and received research support from ADx Neurosciences, Fujirebio, AlzPath Inc., Roche Diagnostics, and Eli Lilly and Company in the past 2 years. Dage has received speaker fees from Eli Lilly and Company. Dage is a founder and advisor for Monument Biosciences. Dage has stock or stock options in Eli Lilly and Company, Genotix Biotechnologies, AlzPath Inc., and Monument Biosciences. Michelle Mielke consults for or serves on advisory boards for Biogen, Eisai, Lilly, Merck, Roche, and Siemens Healthineers. Suzanne Craft reports disclosures for vTv Therapeutics, T3D Therapeutics, Cyclerion Inc., and Cognito Inc. Author disclosures are available in the supporting information.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
- U19AG074879/GF/NIH HHS/United States
- U54 AG065181/AG/NIA NIH HHS/United States
- T32 AG033534/AG/NIA NIH HHS/United States
- P30 AG049638/AG/NIA NIH HHS/United States
- U01AG057195/GF/NIH HHS/United States
- P30AG049638/GF/NIH HHS/United States
- R01 AG058969/AG/NIA NIH HHS/United States
- R01 AG054069/AG/NIA NIH HHS/United States
- P30AG072947/GF/NIH HHS/United States
- P30 AG072976/AG/NIA NIH HHS/United States
- R01 AG072474/AG/NIA NIH HHS/United States
- RD006263/GF/NIH HHS/United States
- R01AG068193/GF/NIH HHS/United States
- U54AG065181/GF/NIH HHS/United States
- U54 AG054345/AG/NIA NIH HHS/United States
- U01AG082350/GF/NIH HHS/United States
- T32AG033534/GF/NIH HHS/United States
- P30 AG010133/AG/NIA NIH HHS/United States
- U24 AG021886/AG/NIA NIH HHS/United States
- U54AG054345/GF/NIH HHS/United States
- U01 AG082350/AG/NIA NIH HHS/United States
- U01 AG057195/AG/NIA NIH HHS/United States
- U24 AG072122/AG/NIA NIH HHS/United States
- U19 AG074879/AG/NIA NIH HHS/United States
- U24 AG082930/AG/NIA NIH HHS/United States
- U24AG082930/GF/NIH HHS/United States
- P30AG072976/GF/NIH HHS/United States
- R01 AG077202/AG/NIA NIH HHS/United States
- MJFF-023365/Michael J Fox Foundation
- R01 AG068193/AG/NIA NIH HHS/United States
- R01AG072474/GF/NIH HHS/United States
- U24AG021886/GF/NIH HHS/United States
- P30 AG072947/AG/NIA NIH HHS/United States
- R01AG077202/GF/NIH HHS/United States
- RD005665/GF/NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

