Influence of Vitamin D Receptor Signalling and Vitamin D on Colonic Epithelial Cell Fate Decisions in Ulcerative Colitis
- PMID: 38747639
- PMCID: PMC11479711
- DOI: 10.1093/ecco-jcc/jjae074
Influence of Vitamin D Receptor Signalling and Vitamin D on Colonic Epithelial Cell Fate Decisions in Ulcerative Colitis
Erratum in
-
Correction to: Influence of Vitamin D Receptor Signalling and Vitamin D on Colonic Epithelial Cell Fate Decisions in Ulcerative Colitis.J Crohns Colitis. 2025 May 8;19(5):jjae168. doi: 10.1093/ecco-jcc/jjae168. J Crohns Colitis. 2025. PMID: 39589829 Free PMC article. No abstract available.
Abstract
Background and aims: Epidemiological studies have shown that subnormal levels of vitamin D (25[OH]D) are associated with a more aggravated clinical course of ulcerative colitis [UC]. Despite an increased focus on the therapeutic importance of vitamin D and vitamin D receptor [VDR] signalling, the mechanisms underlying the effects of the vitamin D-VDR axis on UC remain elusive. Therefore, we aimed to investigate whether exposure to active vitamin D (1,25[OH]2D3/VDR) signalling in human organoids could influence the maintenance of the colonic epithelium.
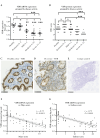
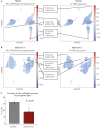
Methods: Intestinal VDR expression was studied by immunohistochemistry, RNA expression arrays, and single-cell RNA sequencing of colonic biopsy specimens obtained from patients with UC and healthy individuals. To characterise the functional and transcriptional effects of 1,25[OH]2D3, we used patient-derived colonic organoids. The dependency of VDR was assessed by knocking out the receptor with CRISPR/Cas9.
Results: Our results suggest that 1,25[OH]2D3/VDR stimulation supports differentiation of the colonic epithelium and that impaired 1,25[OH]2D3/VDR signalling thereby may compromise the structure of the intestinal epithelial barrier, leading to flares of UC. Furthermore, a transcriptional response to VDR activity was observed primarily in fully differentiated cells at the top of the colonic crypt, and this response was reduced during flares of UC.
Conclusions: We identified an important role of vitamin D signalling in supporting differentiated cell states in the human colonic epithelium, and thereby maintenance of the intestinal barrier integrity. This makes the vitamin D-VDR signalling axis an interesting target for therapeutic efforts to achieve and maintain remission in patients with UC.
Keywords: Colonic epithelium; inflammatory bowel disease; vitamin D.
© The Author(s) 2024. Published by Oxford University Press on behalf of European Crohn’s and Colitis Organisation.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures






References
-
- Le Berre C, Honap S, Peyrin-Biroulet L. Ulcerative colitis. Lancet 2023;402:571–84. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

