Real-world outcomes of ponatinib treatment in 724 patients with CML and Ph+ ALL: a post-marketing surveillance study with a special interest in arterial occlusive events in Japan
- PMID: 38747937
- PMCID: PMC11322879
- DOI: 10.1093/jjco/hyae061
Real-world outcomes of ponatinib treatment in 724 patients with CML and Ph+ ALL: a post-marketing surveillance study with a special interest in arterial occlusive events in Japan
Abstract
Background: In September 2016, ponatinib was approved in Japan for the treatment of patients with chronic myeloid leukemia with resistance/intolerance to prior tyrosine kinase inhibitors and patients with relapsed or refractory Philadelphia chromosome-positive acute lymphoblastic leukemia.
Methods: We conducted a post-marketing all-case surveillance to study the safety and efficacy of ponatinib in clinical practice, focusing on arterial occlusive events.
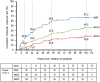
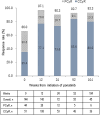
Results: Data from 724 patients were collected for 2 years from the initiation of ponatinib. The arterial occlusive events were reported in 6.49% (47/724) with an exposure-adjusted incidence rate of 6.8/100 person-years. The risks associated with arterial occlusive events were age and comorbidities including hypertension and diabetes. At 104 weeks, the cumulative major molecular response rate in patients with chronic-phase chronic myeloid leukemia was 67.2% and the complete cytogenetic response in patients with Philadelphia chromosome-positive acute lymphoblastic leukemia was 80.0%. Furthermore, the estimated 1-year overall survival rate was 98.5% for chronic-phase chronic myeloid leukemia and 68.6% for Philadelphia chromosome-positive acute lymphoblastic leukemia.
Conclusions: This surveillance demonstrated that ponatinib has a favorable safety and efficacy profile in Japanese patients and also showed the necessity of closely monitoring arterial occlusive events in older adults and patients with predisposing factors for atherosclerosis.
Keywords: acute lymphoblastic leukemia; arterial occlusive events; chronic myeloid leukemia; ponatinib; post-marketing surveillance.
© The Author(s) 2024. Published by Oxford University Press.
Conflict of interest statement
Naoto Takahashi has received all support for the present manuscript from Otsuka Pharmaceutical; honoraria from Pfizer, Novartis and Otsuka Pharmaceutical; Kiyohiko Hatake has received all support for the present manuscript from Otsuka Pharmaceutical; consuling fee from Meiji Seika Pharmaceutical; honoraria from Takeda Pharmaceutical, Celgene Corporation, Eisai, Meiji Seika Pharmaceutical, Chugai Pharmaceutical, towa pharmaceutical, Daiichi Sankyo and Yakult Headquarters; Yuji Ikari has received all support for the present manuscript from Otsuka Pharmaceutical; honoraria from Abbott Medical Japan, Amgen, Japan,Boehringerheim, Bayer Pharmaceuticals, AstraZeneca, Daiichi Sankyo and Novartis; leaderchip or fiduciancy role in other board, survey, committee or advocary group, paid or unpaid; from Japanese circulation society, Japnanese association of caridiovacular intervention and therapeutics; Takeshi Kondo has received all support for the present manuscript from Otsuka Pharmaceutical; grants or contracts from any entity from Pfizer Health Research Foundation; honoraria from Astellas Pharma, Pfizer, Nippon Sinyaku, Eisai, Novartis, Abbvie, Meiji Seika Pharma, Bristol Meyers Squibb, Asahi Kasei Pharma and Otsuka Pharmaceutical; Yushihiro Fukumoto has received all support for the present manuscript from Otsuka Pharmaceutical; grants or contracts from any entity from Alnylam Pharmaceuticals, Janssen Pharmaceutical, lonis Pharmaceutical INC, Japan Medical Device Technology, Bayer Pharmaceuticals, Teijin Pharma, Japan Lifeline and Abbott Medical Japan; honoraria from AstraZeneca, Ono Pharmaceutical, Daiichi Sankyo, Japan,Boehringerheim, Bayel Pharmaceutical, Otsuka Pharmaceutical, Kowa, Toaeiyo, Novartis and Janssen Pharmaceutical; Arinob Tojo have received all support for the present manuscript from Otsuka Pharmaceutical; grants or contracts from any entity from KM Biologics and Sysmex; consulting fee from Sysmex and LSI medicine; honoraria from Otsuka Pharmaceutical, Novartis and Bristol Meyer Squib.Akira Masunari, Seiji Nishibayashi, Akiko Kageyama and Yasuhiko Fukuta are employees of Otsuka Pharmaceutical.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

