Cannabidivarin and cannabigerol induce unfolded protein response and angiogenesis dysregulation in placental trophoblast HTR-8/SVneo cells
- PMID: 38748041
- PMCID: PMC11324689
- DOI: 10.1007/s00204-024-03781-8
Cannabidivarin and cannabigerol induce unfolded protein response and angiogenesis dysregulation in placental trophoblast HTR-8/SVneo cells
Abstract
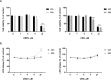
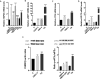
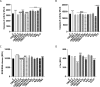
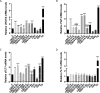
Cannabidivarin (CBDV) and cannabigerol (CBG) are minor phytocannabinoids from Cannabis sativa, whose health benefits have been reported. However, studies about the impact of these cannabinoids on fundamental cellular processes in placentation are scarce. Placental development involves physiological endoplasmic reticulum (ER) stress, however when exacerbated it can lead to altered angiogenesis and pregnancy disorders, such as intrauterine growth restriction and preeclampsia. In this work, the effects of CBDV and CBG (1-10 µM) on placental extravillous trophoblasts were studied, using the in vitro model HTR-8/SVneo cells. Both cannabinoids induced anti-proliferative effects and reactive oxygen/nitrogen species generation, which was dependent on transient receptor potential vanilloid 1 (TRPV1) activation. Moreover, CBDV and CBG significantly upregulated, in a TRPV-1 dependent manner, the gene expression of HSPA5/Glucose-regulated protein 78 (GRP78/BiP), a critical chaperone involved in ER stress and unfolded protein response (UPR) activation. Nevertheless, the UPR pathways were differentially activated. Both cannabinoids were able to recruit the IRE branch, while only CBDV enhanced the expression of downstream effectors of the PERK pathway, namely p-eIF2α, ATF4 and CHOP. It also augmented the activity of the apoptotic initiator caspases-8 and -9, though the effector caspases-3/-7 were not activated. TRB3 expression was increased by CBDV, which may hinder apoptosis termination. Moreover, both compounds upregulated the mRNA levels of the angiogenic factors VEGFA, PGF and sFLT1, and disrupted the endothelial-like behavior of HTR-8/SVneo cells, by reducing tube formation. Thus, CBDV and CBG treatment interferes with EVTs functions and may have a negative impact in placentation and in pregnancy outcome.
Keywords: Angiogenesis; Cannabidivarin; Cannabigerol; Cannabinoids; Endoplasmic reticulum stress; Placenta.
© 2024. The Author(s).
Conflict of interest statement
The authors have no relevant financial or non-financial interests to disclose.
Figures


Similar articles
-
Krüppel-like factor 6 involvement in the endoplasmic reticulum homeostasis of extravillous trophoblasts.Placenta. 2024 Sep 26;155:42-51. doi: 10.1016/j.placenta.2024.08.002. Epub 2024 Aug 5. Placenta. 2024. PMID: 39121586
-
Aβ1-42 stimulates an increase in autophagic activity through tunicamycin-induced endoplasmic reticulum stress in HTR-8/SVneo cells and late-onset pre-eclampsia.J Mol Histol. 2024 Aug;55(4):513-525. doi: 10.1007/s10735-024-10203-7. Epub 2024 May 23. J Mol Histol. 2024. PMID: 38777993
-
[Role of endoplasmic reticulum stress-induced apoptosis of trophoblasts in intrahepatic cholestasis during pregnancy].Nan Fang Yi Ke Da Xue Xue Bao. 2018 May 20;38(5):572-577. doi: 10.3969/j.issn.1673-4254.2018.05.11. Nan Fang Yi Ke Da Xue Xue Bao. 2018. PMID: 29891454 Free PMC article. Chinese.
-
Endoplasmic Reticulum Stress and Cancer: Could Unfolded Protein Response Be a Druggable Target for Cancer Therapy?Int J Mol Sci. 2023 Jan 13;24(2):1566. doi: 10.3390/ijms24021566. Int J Mol Sci. 2023. PMID: 36675080 Free PMC article. Review.
-
TGFβ signalling: a nexus between inflammation, placental health and preeclampsia throughout pregnancy.Hum Reprod Update. 2024 Jul 1;30(4):442-471. doi: 10.1093/humupd/dmae007. Hum Reprod Update. 2024. PMID: 38519450 Free PMC article. Review.
Cited by
-
Maternal Prenatal Cannabis Use and Child Autism Spectrum Disorder.JAMA Netw Open. 2024 Oct 1;7(10):e2440301. doi: 10.1001/jamanetworkopen.2024.40301. JAMA Netw Open. 2024. PMID: 39422906 Free PMC article.
-
The impact of cannabinoids on reproductive function.Reproduction. 2025 Apr 3;169(5):e240369. doi: 10.1530/REP-24-0369. Print 2025 May 1. Reproduction. 2025. PMID: 40111139 Free PMC article. Review.
References
-
- Abcouwer SF, Marjon PL, Loper RK, Vander Jagt DL (2002) Response of VEGF expression to amino acid deprivation and inducers of endoplasmic reticulum stress. Invest Ophthalmol vis Sci 43(8):2791–2798 - PubMed
-
- Amaral C, Trouille FM, Almeida CF, Correia-da-Silva G, Teixeira N (2021) Unveiling the mechanism of action behind the anti-cancer properties of cannabinoids in ER+ breast cancer cells: Impact on aromatase and steroid receptors. J Steroid Biochem Mol Biol 210:105876. 10.1016/j.jsbmb.2021.105876 10.1016/j.jsbmb.2021.105876 - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

