Immunomodulating effects of the single bacterial strain therapy EDP1815 on innate and adaptive immune challenge responses - a randomized, placebo-controlled clinical trial
- PMID: 38748319
- PMCID: PMC11347467
- DOI: 10.1007/s12026-024-09484-7
Immunomodulating effects of the single bacterial strain therapy EDP1815 on innate and adaptive immune challenge responses - a randomized, placebo-controlled clinical trial
Abstract
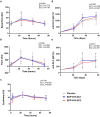
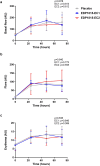
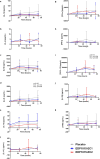
The gut microbiome can modulate systemic inflammation and is therefore target for immunomodulation. Immunomodulating effects of EDP1815, a bacterial commensal strain of Prevotella histicola, were studied in healthy participants. Effects on adaptive immunity were evaluated by a neo-antigen challenge with keyhole limpet haemocyanin (KLH), while effects on innate immunity were evaluated by topical toll-like receptor 7 (TLR7) agonist imiquimod. Capsules with two enteric coating levels (EC1, EC2) were compared. Thirty-six healthy participants were included and received a daily dose of 8 × 1010 cells EDP1815-EC1, EDP1815-EC2 or placebo (randomization 1:1:1) for 60 days. They received KLH vaccinations at days 8, 24 and 36, with intradermal skin challenge at day 57. KLH challenge outcomes were antibody levels, and skin blood flow and erythema after skin challenge, measured by imaging techniques. Imiquimod administration started at day 57, for 72 h. Outcomes consisted of imaging measurements similar to the KLH challenge, and the influx of inflammatory cells and cytokines in blister fluid. There was no effect of EDP1815 treatment on the KLH challenge, neither on the imaging outcomes of the imiquimod challenge. There was a consistently lower influx of inflammatory cells in the blister fluid of EDP1815-treated participants (neutrophils, p = 0.016; granulocytes, p = 0.024), more pronounced in EC1. There was a lower influx of interleukin [IL]-1β, IL-6, IL-8, IL-10, interferon [IFN]-γ and tumour necrosis factor in blister fluid of EDP1815-treated participants. EDP1815 had immunomodulatory effects on the innate immune response driven by imiquimod, but no effect on the KLH challenge was observed. Trial registration number: NCT05682222; date: 22 July 2022.
Keywords: Autoimmune diseases; Imiquimod Immunodermatology; Immune challenges; Keyhole limpet haemocyanin; Microbiome.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Clinical translation of anti-inflammatory effects of Prevotella histicola in Th1, Th2, and Th17 inflammation.Front Med (Lausanne). 2023 May 5;10:1070433. doi: 10.3389/fmed.2023.1070433. eCollection 2023. Front Med (Lausanne). 2023. PMID: 37215725 Free PMC article.
-
Impact of oral administration of single strain Lactococcus lactis spp. cremoris on immune responses to keyhole limpet hemocyanin immunization and gut microbiota: A randomized placebo-controlled trial in healthy volunteers.Front Immunol. 2022 Dec 7;13:1009304. doi: 10.3389/fimmu.2022.1009304. eCollection 2022. Front Immunol. 2022. PMID: 36582231 Free PMC article. Clinical Trial.
-
A randomized, double-blinded, phase 2 trial of EDP1815, an oral immunomodulatory preparation of Prevotella histicola, in adults with mild-to-moderate plaque psoriasis.Front Med (Lausanne). 2024 May 15;11:1292406. doi: 10.3389/fmed.2024.1292406. eCollection 2024. Front Med (Lausanne). 2024. PMID: 38813388 Free PMC article.
-
Interferon and Toll-Like Receptor 7 Response in COVID-19: Implications of Topical Imiquimod for Prophylaxis and Treatment.Dermatology. 2021;237(6):847-856. doi: 10.1159/000518471. Epub 2021 Aug 31. Dermatology. 2021. PMID: 34511591 Free PMC article. Review.
-
Keyhole limpet haemocyanin - a model antigen for human immunotoxicological studies.Br J Clin Pharmacol. 2014 Nov;78(5):1135-42. doi: 10.1111/bcp.12422. Br J Clin Pharmacol. 2014. PMID: 24833186 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

