References for Small Fluorescence Quantum Yields
- PMID: 38748338
- PMCID: PMC12095370
- DOI: 10.1007/s10895-024-03729-2
References for Small Fluorescence Quantum Yields
Abstract
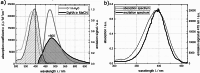
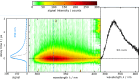
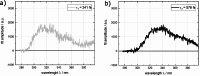
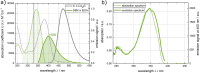
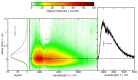
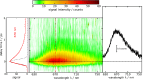
Three compounds with fluorescence quantum yields in the range of 10- 5 to 10- 4 and emission spectra covering the UV/Vis spectral range are suggested as new references for the determination of small fluorescence quantum yields. The compounds are thymidine (dT) in water, dibenzoylmethane (DBM) in ethanol, and malachite green chloride (MG) in water, representing the blue, green, and red regions of the spectrum, respectively. All compounds are easily handled, photostable, and commercially available. Furthermore, these compounds exhibit a mirror-image symmetry between their absorption and fluorescence spectra. This symmetry, along with closely aligned fluorescence excitation and absorption spectra, confirms that the observed emissions originate from the compounds themselves. The fluorescence quantum yields were determined via a relative approach as well as Strickler-Berg analysis in conjunction with time resolved fluorescence spectroscopy. Within the respective error margins, the two approaches yielded identical results.
Keywords: Dibenzoylmethane; Fluorescence quantum yield; Malachite green chloride; Strickler-Berg analysis; Thymidine; Time resolved spectroscopy.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethical Approval: Not applicable. Competing Interests: The authors declare no competing interests.
Figures




References
-
- Wong K-L, Bünzli J-CG, Tanner PA (2020) Quantum yield and brightness. J Lumin 224:117256. 10.1016/j.jlumin.2020.117256
-
- Braslavsky SE (2007) Glossary of terms used in photochemistry, (IUPAC recommendations 2006). Pure Appl Chem 79:293–465. 10.1351/pac200779030293
-
- Birriel JJ, King D (2018) Fluorescence spectra of highlighter inks. Phys Teach 56:20–23. 10.1119/1.5018682
-
- Kalyani NT, Dhoble S (2012) Organic light emitting diodes: Energy saving lighting technology—A review. Renew Sustain Energy Rev 16:2696–2723. 10.1016/j.rser.2012.02.021
LinkOut - more resources
Full Text Sources

