Synergistic induction of blood-brain barrier properties
- PMID: 38748577
- PMCID: PMC11126970
- DOI: 10.1073/pnas.2316006121
Synergistic induction of blood-brain barrier properties
Abstract
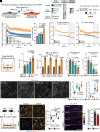
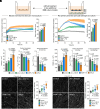
Blood-brain barrier (BBB) models derived from human stem cells are powerful tools to improve our understanding of cerebrovascular diseases and to facilitate drug development for the human brain. Yet providing stem cell-derived endothelial cells with the right signaling cues to acquire BBB characteristics while also retaining their vascular identity remains challenging. Here, we show that the simultaneous activation of cyclic AMP and Wnt/β-catenin signaling and inhibition of the TGF-β pathway in endothelial cells robustly induce BBB properties in vitro. To target this interaction, we present a small-molecule cocktail named cARLA, which synergistically enhances barrier tightness in a range of BBB models across species. Mechanistically, we reveal that the three pathways converge on Wnt/β-catenin signaling to mediate the effect of cARLA via the tight junction protein claudin-5. We demonstrate that cARLA shifts the gene expressional profile of human stem cell-derived endothelial cells toward the in vivo brain endothelial signature, with a higher glycocalyx density and efflux pump activity, lower rates of endocytosis, and a characteristic endothelial response to proinflammatory cytokines. Finally, we illustrate how cARLA can improve the predictive value of human BBB models regarding the brain penetration of drugs and targeted nanoparticles. Due to its synergistic effect, high reproducibility, and ease of use, cARLA has the potential to advance drug development for the human brain by improving BBB models across laboratories.
Keywords: blood–brain barrier; drug delivery; endothelial cell; in vitro models; signaling pathways.
Conflict of interest statement
Competing interests statement:I.K. and M.V. are employed by the company Gedeon Richter. HUN-REN BRC has filed a patent (PCT/HU2023/050070) related to this work; G.P., M.M., A.S., S.V., and M.A.D. are named inventors. Other authors declare no competing interest. Hungarian national patent P2300053 was filed on 9 February 2023 by HUN-REN Biological Research Centre, Szeged, Hungary. Inventors are authors G.P., M.A.D., S.V., M.M., and A.S.
Figures




References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

