Health-related quality of life with sacituzumab govitecan in HR+/HER2- metastatic breast cancer in the phase III TROPiCS-02 trial
- PMID: 38748596
- PMCID: PMC11379636
- DOI: 10.1093/oncolo/oyae088
Health-related quality of life with sacituzumab govitecan in HR+/HER2- metastatic breast cancer in the phase III TROPiCS-02 trial
Erratum in
-
Correction to: Health-related quality of life with sacituzumab govitecan in HR+/HER2- metastatic breast cancer in the phase III TROPiCS-02 trial.Oncologist. 2025 Mar 10;30(3):oyae370. doi: 10.1093/oncolo/oyae370. Oncologist. 2025. PMID: 40132065 Free PMC article. No abstract available.
Abstract
Background: The TROPiCS-02 study (NCT03901339) demonstrated that sacituzumab govitecan (SG) has superior clinical outcomes over treatment of physician's choice (TPC) chemotherapy in patients with hormone receptor-positive, human epidermal growth factor 2 receptor-negative (HR+/HER2-) metastatic breast cancer (mBC). Here, we present health-related quality of life (HRQoL) patient-reported outcome (PRO) findings from this study.
Patients and methods: Eligible adults with HR+/HER2- mBC who previously received a taxane, endocrine-based therapy, a CDK4/6 inhibitor, and 2-4 lines of chemotherapy were randomized 1:1 to receive SG or TPC until progression or unacceptable toxicity. PROs were assessed at baseline and on day 1 of each cycle, using the European Organization for Research and Treatment of Cancer Quality-of-Life Core 30 (EORTC QLQ-C30), EQ-5D-5L, and PRO Common Terminology Criteria for Adverse Events (PRO-CTCAE).
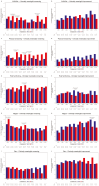
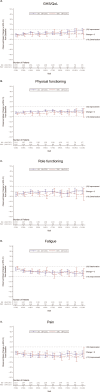
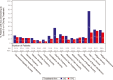
Results: Compared to TPC, overall least square mean change from baseline was significantly better for SG for physical functioning and dyspnea, but worse for diarrhea. Time to first clinically meaningful worsening or death was significantly longer for SG in global health status/quality of life, physical functioning, fatigue, emotional functioning, dyspnea, insomnia, and financial difficulties of the EORTC QLQ-C30 and the EQ-VAS, but longer for TPC in diarrhea. Few patients in both arms reported experiencing any worsening to level 3 or 4 treatment-related symptomatic events during treatment, as assessed by 16 PRO-CTCAE items, except for diarrhea frequency and amount of hair loss, which favored TPC.
Conclusions: SG was associated with an HRQoL benefit in most symptoms and functioning, compared with TPC. This supports the favorable profile of SG as a treatment option for patients with pretreated HR+/HER2- mBC.
Keywords: EORTC QLQ-C30; HR+/HER2−; Sacituzumab govitecan; antibody–drug conjugate; metastatic breast cancer; phase III; quality of life.
© The Author(s) 2024. Published by Oxford University Press.
Conflict of interest statement
H.S.R. reports honoraria from Puma Biotechnology, Mylan, and Samsung Bioepis; and institutional research funding from Macrogenics, OBI Pharma, Pfizer, Novartis, Lilly, Genentech, Merck, Odonate Therapeutics, Daiichi Sankyo, Seattle Genetics, Sermonix Pharmaceuticals, AstraZeneca, Gilead Sciences, and Ayala Pharmaceuticals. P.S. reports advisory board fees from AstraZeneca, Bay, Boehinger Ingelheim, Merck, Novartis, Pfizer, Puma, Roche, Gilead, Eisai, MSD, Seagen, Amgen, Lily, and Celgene; and institutional research grants from Astellas, AstraZeneca, Genentec, Novartis, Oncogenex, Roche, and Medivation. S.M.T. reports grants and personal fees from Immunomedics/Gilead, AstraZeneca, Eli Lilly, Merck, Nektar, Novartis, Pfize, Genentech/Roche, Exelixis, BMS, Eisai, NanoString, Sanofi, Odonate, and Immunomedics/Gilead; personal fees fom Puma, Celldex, Seattle Genetics, Silverback Therapeutics, G1 Therapeutics, AbbVie, Athenex, OncoPep, Kyowa Kirin Pharmaceuticals, Daiichi Sankyo, CytomX, Samsung Bioepis Inc., Certara, Mersana Therapeutics; and grants from Cyclacel. F.D. has declared no conflicts of interest. F.M. reports institutional research funding from Roche, Novartis, AstraZeneca, GSK/Tesaro, MED, Clovis, Vaccibody, Gilead Sciences, and Eisai; consulting fees from AstraZeneca, TESARO/GSK, Pfizer, Eisai, Gilead, Vaccibody, and GenomicHealth; honoraria from AstraZeneca, Clovis, GSK/Tesaro, Eli Lilly, Novartis, Pfizer, Roche, Myriad Genetics, PharmaMar, Eisai, MSD, Immunomedics/Gilead, Pierre-Fabre, Agendia, Genomic Health, and Seattle Genetics; support for meeting attendance/travel from Pfizer, Roche, and AstraZeneca; and data safety monitoring board or advisory board fees from Palleos and Amgen. L.S. reports employment by Evidera. W.V. reports employment by Gilead Sciences, Inc. A.S. reports employment by Gilead Sciences, Inc.; and stock ownership in Roche and Gilead Sciences, Inc. M.G. reports employment by and stock ownership in Gilead Sciences, Inc. A.B. reports institutional research funding from Genentech, Novartis, Pfizer, Merck, Sanofi, Radius Health/Menarini, Immunomedics/Gilead, Daiichi Pharma/AstraZeneca, and Eli Lilly; and consulting fees from Pfizer, Novartis, Genentech, Merck, Radius Health/Menarini, Immunomedics/Gilead, Sanofi, Daiichi Pharma/AstraZeneca, Phillips, Eli Lilly, and Foundation Medicine. J.C. reports institutional research funding from Roche, Ariad Pharmaceuticals, AstraZeneca, Baxalta, GMBH/Servier Affaires, Bayer HealthCare, Eisai, F. Hoffman-La Roche, Guardant Health, Merck Sharp & Dohme, Pfizer, Piqur Therapeutics, Puma C, Queen Mary University of London; consulting fees from Roche, Celgene, Cellestia, AstraZeneca, Seattle Genetics, Daiichi Sankyo, Erytech, Athenex, Polyphor, Lilly, Merck, Sharp & Dohme, GSK, Leuko, Bioasis, Clovis Oncology, Boehringer Ingelheim, Ellipses, Hibercell, BioInvent, GEMoaB, Gilead, Menarini, Zymeworks, Reveal Genomics, and Expres2ion Biotechnologies; honoraria from Roche, Novartis, Celgene, Eisai, Pfizer, Samsung Bioepis, Lilly, Merck, Sharp & Dohme, and Daiichi Sankyo; travel and accommodations from Roche, Novartis, Eisai, Pfizer, Daiichi Sankyo, AstraZeneca, and Gilead Sciences; and stock ownership from MedSIR, and Nektar Pharmaceuticals; and multiple patents.
Figures
References
-
- American Cancer Society. Breast cancer facts & figures 2022-2024. – [accessed: January 24, 2024]. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-....
-
- Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Breast Cancer V2.2024. © National Comprehensive Cancer Network, Inc. 2024. All rights reserved. Accessed March 14, 2024. To view the most recent and complete version of the guideline, go online to NCCN.org. NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

