Effects of ozone exposure on lung injury, inflammation, and oxidative stress in a murine model of nonpneumonic endotoxemia
- PMID: 38749002
- PMCID: PMC11285192
- DOI: 10.1093/toxsci/kfae062
Effects of ozone exposure on lung injury, inflammation, and oxidative stress in a murine model of nonpneumonic endotoxemia
Abstract
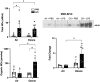
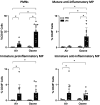
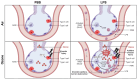
Recent studies have identified exposure to environmental levels of ozone as a risk factor for the development of acute respiratory distress syndrome (ARDS), a severe form of acute lung injury (ALI) that can develop in humans with sepsis. The aim of this study was to develop a murine model of ALI to mechanistically explore the impact of ozone exposure on ARDS development. Mice were exposed to ozone (0.8 ppm, 3 h) or air control followed 24 h later by intravenous administration of 3 mg/kg lipopolysaccharide (LPS) or PBS. Exposure of mice to ozone + LPS caused alveolar hyperplasia; increased BAL levels of albumin, IgM, phospholipids, and proinflammatory mediators including surfactant protein D and soluble receptor for advanced glycation end products were also detected in BAL, along with markers of oxidative and nitrosative stress. Administration of ozone + LPS resulted in an increase in neutrophils and anti-inflammatory macrophages in the lung, with no effects on proinflammatory macrophages. Conversely, the numbers of resident alveolar macrophages decreased after ozone + LPS; however, expression of Nos2, Arg1, Cxcl1, Cxcl2, Ccl2 by these cells increased, indicating that they are activated. These findings demonstrate that ozone sensitizes the lung to respond to endotoxin, resulting in ALI, oxidative stress, and exacerbated pulmonary inflammation, and provide support for the epidemiologic association between ozone exposure and ARDS incidence.
Keywords: acute lung injury; inflammation; oxidative stress; ozone; sepsis.
© The Author(s) 2024. Published by Oxford University Press on behalf of the Society of Toxicology. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures








References
-
- Atochina E. N., Beers M. F., Hawgood S., Poulain F., Davis C., Fusaro T., Gow A. J. (2004). Surfactant protein-D, a mediator of innate lung immunity, alters the products of nitric oxide metabolism. Am. J. Respir. Cell Mol. Biol. 30, 271–279. - PubMed
-
- Bellani G., Laffey J. G., Pham T., Fan E., Brochard L., Esteban A., Gattinoni L., van Haren F., Larsson A., McAuley D. F., et al.; ESICM Trials Group. (2016). Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA 315, 788–800. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

