Long-term results of the sequential combination of cladribine and rituximab in Hairy cell leukemia
- PMID: 38749022
- PMCID: PMC11646483
- DOI: 10.1080/10428194.2024.2349700
Long-term results of the sequential combination of cladribine and rituximab in Hairy cell leukemia
Abstract
We report on the long-term efficacy and safety of a phase 2 trial of sequential cladribine and rituximab in hairy cell leukemia (HCL). One-hundred and thirty-nine patients were enrolled: 111 in the frontline setting, 18 in first relapse, and 10 with variant HCL (HCLv). A complete response (CR) was achieved in 133 of 137 evaluable participants (97%) with measurable residual disease (MRD) negativity in 102 (77%). MRD status was not associated with significant differences in event-free survival (EFS) or overall survival (OS). With a median follow-up of 7.8 years (range: 0.40-18.8), eight patients have experienced disease relapse (5.8%), 4/111 with newly diagnosed HCL (3·6%) and 4/10 with HCLv (40%) (p = 0.002). The 10-year EFS and OS rates were 86.7% and 91.1%, respectively. Grade 3 adverse events were observed in 28 participants (20·1%), mostly due to infections. Treatment of HCL with sequential cladribine followed by rituximab is associated with excellent efficacy and safety results both in the frontline and relapsed settings.
Keywords: HCL; HCLv; cladribine; clinical trial; rituximab.
Conflict of interest statement
Figures


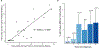
References
-
- Kraut EH. Clinical manifestations and infectious complications of hairy-cell leukaemia. Vol. 16, Best Practice and Research: Clinical Haematology. 2003. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
