CEA-CD3 bispecific antibody cibisatamab with or without atezolizumab in patients with CEA-positive solid tumours: results of two multi-institutional Phase 1 trials
- PMID: 38750034
- PMCID: PMC11096172
- DOI: 10.1038/s41467-024-48479-8
CEA-CD3 bispecific antibody cibisatamab with or without atezolizumab in patients with CEA-positive solid tumours: results of two multi-institutional Phase 1 trials
Abstract
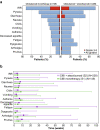
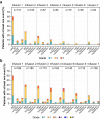
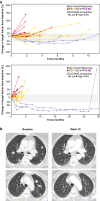
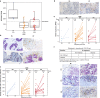
Cibisatamab is a bispecific antibody-based construct targeting carcinoembryonic antigen (CEA) on tumour cells and CD3 epsilon chain as a T-cell engager. Here we evaluated cibisatamab for advanced CEA-positive solid tumours in two open-label Phase 1 dose-escalation and -expansion studies: as a single agent with or without obinutuzumab in S1 (NCT02324257) and with atezolizumab in S2 (NCT02650713). Primary endpoints were safety, dose finding, and pharmacokinetics in S1; safety and dose finding in S2. Secondary endpoints were anti-tumour activity (including overall response rate, ORR) and pharmacodynamics in S1; anti-tumour activity, pharmacodynamics and pharmacokinetics in S2. S1 and S2 enrolled a total of 149 and 228 patients, respectively. Grade ≥3 cibisatamab-related adverse events occurred in 36% of S1 and 49% of S2 patients. The ORR was 4% in S1 and 7% in S2. In S2, patients with microsatellite stable colorectal carcinoma (MSS-CRC) given flat doses of cibisatamab and atezolizumab demonstrated an ORR of 14%. In S1 and S2, 40% and 52% of patients, respectively, developed persistent anti-drug antibodies (ADAs). ADA appearance could be mitigated by obinutuzumab-pretreatment, with 8% of patients having persistent ADAs. Overall, cibisatamab warrants further exploration in immunotherapy combination strategies for MSS-CRC.
© 2024. The Author(s).
Conflict of interest statement
NHS reports research funding from Regeneron, Immunocore, Puretech, AstraZeneca, BMS, Merck, Pfizer, Roche/Genentech; served as an consultant/advisory board for Immunocore, PsiOxus, Roche/Genentech, Boehringer Ingelheim, Revitope, ABL Bio, Novartis, GSK, Astra Zeneca, Numab. IM reports grants from Roche, Bristol Myers, AstraZeneca, Genmab, Highlight therapeutics; consultancy fees from Roche, Bristol Myers, AstraZeneca, Genmab, Highlights therapeutics, Numab, F-Star, Catalym, Pharmamamar, Biolinerx, Gossamer, Bright peak, Pieris, Alligator, Pierre FabreVM reports consulting fees from: Roche, Bayer, BMS, Janssen and Basilea; Principal Investigator – Institutional Funding: AbbVie, AceaBio, Adaptimmune, ADC Therapeutics, Aduro, Agenus, Amcure, Amgen, Astellas, AstraZeneca Bayer Beigene BioInvent International AB, BMS, Boehringer, Boheringer, Boston, Celgene, Daichii Sankyo, DEBIOPHARM,Eisai, e-Terapeutics, Exelisis, Forma Therapeutics, Genmab, GSK, Harpoon, Hutchison, Immutep, Incyte, Inovio, Iovance, Janssen, Kyowa Kirin, Lilly, Loxo, MedSir, Menarini, Merck, Merus, Millennium, MSD, Nanobiotix, Nektar, Novartis, Odonate Therapeutics, Pfizer, Pharma Mar, PharmaMar, Principia, PsiOxus, Puma, Regeneron, Rigontec, Roche, Sanofi, Sierra Oncology, Synthon, Taiho, Takeda, Tesaro, Transgene, Turning Point Therapeutics, Upshersmith.. NS reports provided consultation or attended advisory boards for Boehringer Ingelheim, Ellipses Pharma; received research grants for the institute from AB Science, Abbvie, Actuate Therapeutics, ADCtherapeutics, Amgen, Array, Ascendis Pharma, Astex, AstraZeneca, Bayer, Blueprint Medicines, Boehringer Ingelheim, BridgeBio, Bristol-Myers Squibb, Cantargia, Celgene, CellCentric, Cresecendo, Cytovation, Deciphera, Eli Lilly, Exelixis, Genentech, Genmab, Gilead, GlaxoSmithKline, Incyte, InteRNA, Janssen/Johnson&Johnson, Kinate, Merck, Merck Sharp & Dohme, Merus, Molecular Partners, Novartis, Numab, Pfizer, Pierre Fabre, Regeneron, Roche, Sanofi, Seattle Genetics, Servier, Taiho, Takeda (outside the submitted work). AM over the last 5 years received honoraria and travel expense coverage for participation to Roche and Genentech Scientific Advisory Boards although not in direct relationship with the content of the manuscript. KSR reports compensation for conduction of clinical trial from Lilly, Roche/Genentech, Bristol-Myers Squibb, Symphogen, Pfizer, Novartis, Bayer, Alligator Bioscience, Incyte, Genmab, Puma Biotechnology, Orion Clinical, Bioinvent, Monta Bioscience, AstraZeneca; Speaker fees from Bayer, Amgen; Travel and conference reimbursement from AstraZeneca. MERR reports receiving research funding from Roche and Highlight Therapeutics. She also has received speaker’s bureau honoraria from BMS and ROCHE. JE has nothing to disclose. CE reports research support for this trial provided to Vanderbilt-Ingram Cancer Center. GAM reports research funding from Regeneron, BioLineRx, Merck, Roche/Genentech; served on the advisory for CEND Pharmaceuticals, Roche/Genentech; owns stock in CEND Pharmaceuticals. DW is an employee of F. Hoffmann-La Roche. BL has nothing to disclose. SB owns stock in Roche. NF is an employee of F. Hoffmann-La Roche. MDT is an employee of F. Hoffmann-La Roche. ME is an employee of and owns stock in F. Hoffmann-La Roche AG. HK is an employee of Genentech, Inc. and owns stock in F. Hoffmann-La Roche, Ltd. CJ is an employee of and owns stock in F. Hoffmann-La Roche, Ltd. MMF reports Roche profit participation certificate. CL has nothing to disclose. EC reports payment or honoraria from HM Hospitales Group; research funding form START; leadership role at start; consulting for Nanobiotix, Janssen-Cilag, Roche/Genentech, TargImmune Therapeutics, Bristol-Myers Squibb, Amunix, Adcendo, Anaveon, AstraZeneca/MedImmune, Chugai Pharma, MonTa, MDS Oncology, Nouscom, Novartis, Oncology, PharmaMar; employee of START, HM Hospitales Group; owns in START and Oncoart Associate; serves as president and found of foundation INTHEOS (Investigational Therapeutics in Oncological Sciences). LPA reports payment or honoraria from AstraZeneca, Janssen, Merck, Mirati; consulting fees from Lilly, MSD, Roche, Pharmamar, Merck, AstraZeneca, Novartis, Servier, Amgen, Pfizer, Sanofi, bayer, BMS, Mirati, GSK, Janssen, Takeda; research grants from MSD, AstraZeneca, Pfizer, BMS, member of board of directros for Altum Sequencing and Genomica; principle investigator for Alkermes, Amgen, AstraZeneca, Bristol Myers Squibb, Daiichi Sankyo, IO Biotech, Janssen-Cilag, Lilly, MSD, Novartis, Pfizer, Pharmamar, Roche, Sanofi, Takeda, and Tesaro. JT reports personal financial interest in form of scientific consultancy role for Array Biopharma, AstraZeneca, Avvinity, Bayer, Boehringer Ingelheim, Chugai, Daiichi Sankyo, F. Hoffmann-La Roche Ltd, Genentech Inc, HalioDX SAS, Hutchison MediPharma International, Ikena Oncology, Inspirna Inc, IQVIA, Lilly, Menarini, Merck Serono, Merus, MSD, Mirati, Neophore, Novartis, Ona Therapeutics, Orion Biotechnology, Peptomyc, Pfizer, Pierre Fabre, Samsung Bioepis, Sanofi, Seattle Genetics, Scandion Oncology, Servier, Sotio Biotech, Taiho,Tessa Therapeutics and TheraMyc. And also educational collaboration with Imedex, Medscape Education, MJH Life Sciences, PeerView Institute for Medical Education and Physicians Education Resource (PER); declares institutional financial interest in form of financial support for clinical trials or contracted research for Amgen Inc, Array Biopharma Inc,AstraZeneca Pharmaceuticals LP, BeiGene, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Debiopharm International SA, F. Hoffmann-La Roche Ltd, Genentech Inc, HalioDX SAS, Hutchison MediPharma International, Janssen-Cilag SA, MedImmune, Menarini, Merck Health KGAA, Merck Sharp & Dohme, Merus NV, Mirati, Novartis Farmacéutica SA, Pfizer, Pharma Mar, Sanofi Aventis Recherche & Développement, Servier, Taiho Pharma USA Inc, Spanish Association Against Cancer Scientific Foundation and Cancer Research UK. GA reports travel support from Amgen; speaker fees from Amgen, serves as medical advisor for Gadeta BV.
Figures

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

