Three-dimensional assessment of image distortion induced by active cardiac implants in 3.0T CMR
- PMID: 38750100
- PMCID: PMC11096309
- DOI: 10.1038/s41598-024-61283-0
Three-dimensional assessment of image distortion induced by active cardiac implants in 3.0T CMR
Abstract
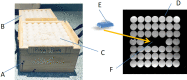
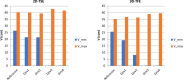
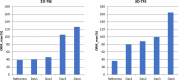
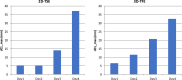
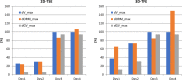
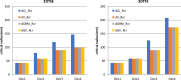
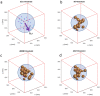
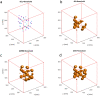
CMR at 3.0T in the presence of active cardiac implants remains a challenge due to susceptibility artifacts. Beyond a signal void that cancels image information, magnetic field inhomogeneities may cause distorted appearances of anatomical structures. Understanding influencing factors and the extent of distortion are a first step towards optimizing the image quality of CMR with active implants at 3.0T. All measurements were obtained at a clinical 3.0T scanner. An in-house designed phantom with a 3D cartesian grid of water filled spheres was used to analyze the distortion caused by four representative active cardiac devices (cardiac loop recorder, pacemaker, 2 ICDs). For imaging a gradient echo (3D-TFE) sequence and a turbo spin echo (2D-TSE) sequence were used. The work defines metrics to quantify the different features of distortion such as changes in size, location and signal intensity. It introduces a specialized segmentation technique based on a reaction-diffusion-equation. The distortion features are dependent on the amount of magnetic material in the active implants and showed a significant increase when measured with the 3D TFE compared to the 2D TSE. This work presents a quantitative approach for the evaluation of image distortion at 3.0T caused by active cardiac implants and serves as foundation for both further optimization of sequences and devices but also for planning of imaging procedures.
Keywords: Active implants; Artifacts; CMR; Distortion; Susceptibility.
© 2024. The Author(s).
Conflict of interest statement
Dr. Weiss is an employee of BIOTRONIK SE & Co. KG, Berlin, Germany, and Dr. Weber is an employee of Philips GmbH, Hamburg, Germany. Prof. Bauer is a scientific advisor for BIOTRONIK SE & Co. KG, Berlin, Germany. Also, this work has been partially funded by BIOTRONIK SE & Co. KG, Berlin, Germany. Dr. Reiter has no competing interests to declare.
Figures
References
-
- Schulz-Menger J, Bluemke DA, Bremerich J, Flamm SD, Fogel MA, Friedrich MG, et al. Standardized image interpretation and post-processing in cardiovascular magnetic resonance - 2020 update: Society for cardiovascular magnetic resonance (SCMR): Board of trustees task force on standardized post-processing. J. Cardiovasc. Magn. Reson. 2020;22(1):19. doi: 10.1186/s12968-020-00610-6. - DOI - PMC - PubMed
-
- Raatikainen MJP, Arnar DO, Merkely B, Nielsen JC, Hindricks G, Heidbuchel H, et al. A decade of information on the use of cardiac implantable electronic devices and interventional electrophysiological procedures in the European Society of Cardiology Countries: 2017 Report from the European Heart Rhythm Association. Europace. 2017;19(suppl_2):ii1–ii90. doi: 10.1093/europace/eux258. - DOI - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

