An immunohistochemical atlas of necroptotic pathway expression
- PMID: 38750308
- PMCID: PMC11250867
- DOI: 10.1038/s44321-024-00074-6
An immunohistochemical atlas of necroptotic pathway expression
Abstract
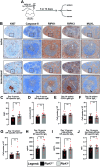
Necroptosis is a lytic form of regulated cell death reported to contribute to inflammatory diseases of the gut, skin and lung, as well as ischemic-reperfusion injuries of the kidney, heart and brain. However, precise identification of the cells and tissues that undergo necroptotic cell death in vivo has proven challenging in the absence of robust protocols for immunohistochemical detection. Here, we provide automated immunohistochemistry protocols to detect core necroptosis regulators - Caspase-8, RIPK1, RIPK3 and MLKL - in formalin-fixed mouse and human tissues. We observed surprising heterogeneity in protein expression within tissues, whereby short-lived immune barrier cells were replete with necroptotic effectors, whereas long-lived cells lacked RIPK3 or MLKL expression. Local changes in the expression of necroptotic effectors occurred in response to insults such as inflammation, dysbiosis or immune challenge, consistent with necroptosis being dysregulated in disease contexts. These methods will facilitate the precise localisation and evaluation of necroptotic signaling in vivo.
Keywords: IBD; Immunohistochemistry; MLKL; Necroptosis; RIPK3.
© 2024. The Author(s).
Figures










References
-
- Alvarez-Diaz S, Dillon CP, Lalaoui N, Tanzer MC, Rodriguez DA, Lin A, Lebois M, Hakem R, Josefsson EC, O’Reilly LA, et al. The pseudokinase MLKL and the kinase RIPK3 have distinct roles in autoimmune disease caused by loss of death-receptor-induced apoptosis. Immunity. 2016;45:513–526. doi: 10.1016/j.immuni.2016.07.016. - DOI - PMC - PubMed
-
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B. 1995;57:289–300. doi: 10.1111/j.2517-6161.1995.tb02031.x. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

