Overexpression of pathogenic tau in astrocytes causes a reduction in AQP4 and GLT1, an immunosuppressed phenotype and unique transcriptional responses to repetitive mild TBI without appreciable changes in tauopathy
- PMID: 38750510
- PMCID: PMC11096096
- DOI: 10.1186/s12974-024-03117-4
Overexpression of pathogenic tau in astrocytes causes a reduction in AQP4 and GLT1, an immunosuppressed phenotype and unique transcriptional responses to repetitive mild TBI without appreciable changes in tauopathy
Abstract
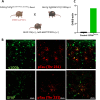
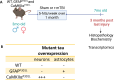
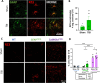
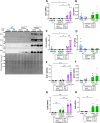
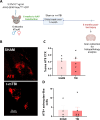
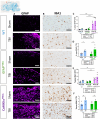
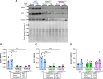
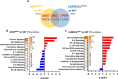
Epidemiological studies have unveiled a robust link between exposure to repetitive mild traumatic brain injury (r-mTBI) and elevated susceptibility to develop neurodegenerative disorders, notably chronic traumatic encephalopathy (CTE). The pathogenic lesion in CTE cases is characterized by the accumulation of hyperphosphorylated tau in neurons around small cerebral blood vessels which can be accompanied by astrocytes that contain phosphorylated tau, the latter termed tau astrogliopathy. However, the contribution of tau astrogliopathy to the pathobiology and functional consequences of r-mTBI/CTE or whether it is merely a consequence of aging remains unclear. We addressed these pivotal questions by utilizing a mouse model harboring tau-bearing astrocytes, GFAPP301L mice, subjected to our r-mTBI paradigm. Despite the fact that r-mTBI did not exacerbate tau astrogliopathy or general tauopathy, it increased phosphorylated tau in the area underneath the impact site. Additionally, gene ontology analysis of tau-bearing astrocytes following r-mTBI revealed profound alterations in key biological processes including immunological and mitochondrial bioenergetics. Moreover, gene array analysis of microdissected astrocytes accrued from stage IV CTE human brains revealed an immunosuppressed astroglial phenotype similar to tau-bearing astrocytes in the GFAPP301L model. Additionally, hippocampal reduction of proteins involved in water transport (AQP4) and glutamate homeostasis (GLT1) was found in the mouse model of tau astrogliopathy. Collectively, these findings reveal the importance of understanding tau astrogliopathy and its role in astroglial pathobiology under normal circumstances and following r-mTBI. The identified mechanisms using this GFAPP301L model may suggest targets for therapeutic interventions in r-mTBI pathogenesis in the context of CTE.
Keywords: Astrocytes; Chronic traumatic encephalopathy; Neuroinflammation; Tau astrogliopathy; Traumatic brain injury.
© 2024. The Author(s).
Conflict of interest statement
The authors declare they have no competing interests.
Figures
Similar articles
-
Evidence for accelerated tauopathy in the retina of transgenic P301S tau mice exposed to repetitive mild traumatic brain injury.Exp Neurol. 2015 Nov;273:168-76. doi: 10.1016/j.expneurol.2015.08.014. Epub 2015 Aug 24. Exp Neurol. 2015. PMID: 26311071
-
Tau immunophenotypes in chronic traumatic encephalopathy recapitulate those of ageing and Alzheimer's disease.Brain. 2020 May 1;143(5):1572-1587. doi: 10.1093/brain/awaa071. Brain. 2020. PMID: 32390044 Free PMC article.
-
Space-occupying brain lesions, trauma-related tau astrogliopathy, and ARTAG: a report of two cases and a literature review.Acta Neuropathol Commun. 2021 Mar 23;9(1):49. doi: 10.1186/s40478-021-01152-3. Acta Neuropathol Commun. 2021. PMID: 33757579 Free PMC article. Review.
-
Chronic Repetitive Mild Traumatic Brain Injury Results in Reduced Cerebral Blood Flow, Axonal Injury, Gliosis, and Increased T-Tau and Tau Oligomers.J Neuropathol Exp Neurol. 2016 Jul;75(7):636-55. doi: 10.1093/jnen/nlw035. Epub 2016 May 31. J Neuropathol Exp Neurol. 2016. PMID: 27251042 Free PMC article.
-
The Neuropathology of Chronic Traumatic Encephalopathy: The Status of the Literature.Semin Neurol. 2020 Aug;40(4):359-369. doi: 10.1055/s-0040-1713632. Epub 2020 Jul 26. Semin Neurol. 2020. PMID: 32712946 Review.
Cited by
-
Covert cerebrospinal fluid dynamics dysfunction: evolution from conventional to innovative therapies.Front Neurol. 2025 Mar 12;16:1554813. doi: 10.3389/fneur.2025.1554813. eCollection 2025. Front Neurol. 2025. PMID: 40144621 Free PMC article. Review.
-
Akkermansia mono-colonization modulates microglia and astrocytes in a strain specific manner.J Neuroinflammation. 2025 Mar 27;22(1):94. doi: 10.1186/s12974-025-03417-3. J Neuroinflammation. 2025. PMID: 40148962 Free PMC article.
-
Extracellular PHF-tau modulates astrocyte mitochondrial dynamics and mediates neuronal connectivity.Transl Neurodegener. 2025 Mar 24;14(1):13. doi: 10.1186/s40035-025-00474-9. Transl Neurodegener. 2025. PMID: 40122883 Free PMC article.
-
Repetitive Low-Level Blast Exposure Alters Circulating Myeloperoxidase, Matrix Metalloproteinases, and Neurovascular Endothelial Molecules in Experienced Military Breachers.Int J Mol Sci. 2025 Feb 20;26(5):1808. doi: 10.3390/ijms26051808. Int J Mol Sci. 2025. PMID: 40076437 Free PMC article.
References
MeSH terms
Substances
Grants and funding
- R56 AG072599/AG/NIA NIH HHS/United States
- P01 AG014449/AG/NIA NIH HHS/United States
- R01 AG074004/AG/NIA NIH HHS/United States
- R01 AG072599/AG/NIA NIH HHS/United States
- W81XWH-19-1-0535/U.S. Department of Defense
- P01 AG017617/AG/NIA NIH HHS/United States
- AG014449/National Institutes of Health,United States
- AG107617/National Institutes of Health,United States
- 13685123/U.S. Department of Defense
- AG077103/National Institutes of Health,United States
- RF1 AG077103/AG/NIA NIH HHS/United States
- AG074004/National Institutes of Health,United States
- AG072599/National Institutes of Health,United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

