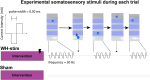
Enhancing touch sensibility with sensory electrical stimulation and sensory retraining
- PMID: 38750521
- PMCID: PMC11096118
- DOI: 10.1186/s12984-024-01371-4
Enhancing touch sensibility with sensory electrical stimulation and sensory retraining
Abstract
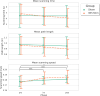
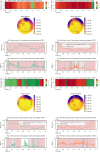
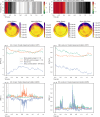
A large proportion of stroke survivors suffer from sensory loss, negatively impacting their independence, quality of life, and neurorehabilitation prognosis. Despite the high prevalence of somatosensory impairments, our understanding of somatosensory interventions such as sensory electrical stimulation (SES) in neurorehabilitation is limited. We aimed to study the effectiveness of SES combined with a sensory discrimination task in a well-controlled virtual environment in healthy participants, setting a foundation for its potential application in stroke rehabilitation. We employed electroencephalography (EEG) to gain a better understanding of the underlying neural mechanisms and dynamics associated with sensory training and SES. We conducted a single-session experiment with 26 healthy participants who explored a set of three visually identical virtual textures-haptically rendered by a robotic device and that differed in their spatial period-while physically guided by the robot to identify the odd texture. The experiment consisted of three phases: pre-intervention, intervention, and post-intervention. Half the participants received subthreshold whole-hand SES during the intervention, while the other half received sham stimulation. We evaluated changes in task performance-assessed by the probability of correct responses-before and after intervention and between groups. We also evaluated differences in the exploration behavior, e.g., scanning speed. EEG was employed to examine the effects of the intervention on brain activity, particularly in the alpha frequency band (8-13 Hz) associated with sensory processing. We found that participants in the SES group improved their task performance after intervention and their scanning speed during and after intervention, while the sham group did not improve their task performance. However, the differences in task performance improvements between groups only approached significance. Furthermore, we found that alpha power was sensitive to the effects of SES; participants in the stimulation group exhibited enhanced brain signals associated with improved touch sensitivity likely due to the effects of SES on the central nervous system, while the increase in alpha power for the sham group was less pronounced. Our findings suggest that SES enhances texture discrimination after training and has a positive effect on sensory-related brain areas. Further research involving brain-injured patients is needed to confirm the potential benefit of our solution in neurorehabilitation.
Keywords: Alpha Power; Electroencephalography; Electrostimulation; Robotic neurorehabilitation; Sensory training; Virtual reality.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures