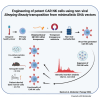
Engineering of potent CAR NK cells using non-viral Sleeping Beauty transposition from minimalistic DNA vectors
- PMID: 38751112
- PMCID: PMC11287004
- DOI: 10.1016/j.ymthe.2024.05.022
Engineering of potent CAR NK cells using non-viral Sleeping Beauty transposition from minimalistic DNA vectors
Abstract
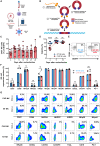
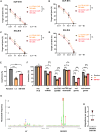
Natural killer (NK) cells have high intrinsic cytotoxic capacity, and clinical trials have demonstrated their safety and efficacy for adoptive cancer therapy. Expression of chimeric antigen receptors (CARs) enhances NK cell target specificity, with these cells applicable as off-the-shelf products generated from allogeneic donors. Here, we present for the first time an innovative approach for CAR NK cell engineering employing a non-viral Sleeping Beauty (SB) transposon/transposase-based system and minimized DNA vectors termed minicircles. SB-modified peripheral blood-derived primary NK cells displayed high and stable CAR expression and more frequent vector integration into genomic safe harbors than lentiviral vectors. Importantly, SB-generated CAR NK cells demonstrated enhanced cytotoxicity compared with non-transfected NK cells. A strong antileukemic potential was confirmed using established acute lymphocytic leukemia cells and patient-derived primary acute B cell leukemia and lymphoma samples as targets in vitro and in vivo in a xenograft leukemia mouse model. Our data suggest that the SB-transposon system is an efficient, safe, and cost-effective approach to non-viral engineering of highly functional CAR NK cells, which may be suitable for cancer immunotherapy of leukemia as well as many other malignancies.
Keywords: CAR; CD19; NK cells; Sleeping Beauty; acute lymphoblastic leukemia; allogenic; non-viral; transposon.
Copyright © 2024. Published by Elsevier Inc.
Conflict of interest statement
Declaration of interests E.U. is an Advisory Board member for Phialogics and has sponsored research projects with Gilead and BMS. Z.I. is an inventor on patents related to Sleeping Beauty and MC technology. M.H. is listed as inventor on patent applications and granted patents related to CAR technologies and transposon-based gene transfer that are, in part licensed to industry. M.H. is a co-founder and equity owner of T-CURX GmbH, Würzburg, Germany. M.H. and E.U. are inventors on patents related to CAR and MC technology. T.B., P.W., W.S.W., and E.U. are inventors on patents related to optimized CAR designs. T.B., P.W., and E.U. are inventors on non-viral gene-editing technologies of NK cells.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

