Gastrointestinal Incomplete Degradation Exacerbates Neurotoxic Effects of PLA Microplastics via Oligomer Nanoplastics Formation
- PMID: 38751156
- PMCID: PMC11267364
- DOI: 10.1002/advs.202401009
Gastrointestinal Incomplete Degradation Exacerbates Neurotoxic Effects of PLA Microplastics via Oligomer Nanoplastics Formation
Abstract
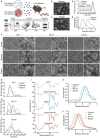
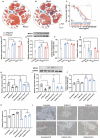
Biodegradable plastics, hailed for their environmental friendliness, may pose unforeseen risks as they undergo gastrointestinal degradation, forming oligomer nanoplastics. Despite this, the influence of gastrointestinal degradation on the potential human toxicity of biodegradable plastics remains poorly understood. To this end, the impact of the murine in vivo digestive system is investigated on the biotransformation, biodistribution, and toxicity of PLA polymer and PLA oligomer MPs. Through a 28-day repeated oral gavage study in mice, it is revealed that PLA polymer and oligomer microplastics undergo incomplete and complete degradation, respectively, in the gastrointestinal tract. Incompletely degraded PLA polymer microplastics transform into oligomer nanoplastics, heightening bioavailability and toxicity, thereby exacerbating overall toxic effects. Conversely, complete degradation of PLA oligomer microplastics reduces bioavailability and mitigates toxicity, offering a potential avenue for toxicity reduction. Additionally, the study illuminates shared targets and toxicity mechanisms in Parkinson's disease-like neurotoxicity induced by both PLA polymer and PLA oligomer microplastics. This involves the upregulation of MICU3 in midbrains, leading to neuronal mitochondrial calcium overload. Notably, neurotoxicity is mitigated by inhibiting mitochondrial calcium influx with MCU-i4 or facilitating mitochondrial calcium efflux with DBcAMP in mice. These findings enhance the understanding of the toxicological implications of biodegradable microplastics on human health.
Keywords: Parkinson's disease; biodistribution; in vivo degradation; mitochondrial calcium uptake family member 3; oligomer nanoplastics.
© 2024 The Authors. Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Kim M. S., Chang H., Zheng L., Yan Q., Pfleger B. F., Klier J., Nelson K., Majumder E. L. W., Huber G. W., Chem. Rev. 2023, 123, 9915. - PubMed
-
- Goel V., Luthra P., Kapur G. S., Ramakumar S. S. V., J. Polym. Environ. 2021, 29, 3079.
-
- Qin M., Chen C., Song B., Shen M., Cao W., Yang H., Zeng G., Gong J., J Clean Prod 2021, 312, 127816.
MeSH terms
Substances
Grants and funding
- 82273656/National Natural Science Foundation of China
- 82304177/National Natural Science Foundation of China
- 82073519/National Natural Science Foundation of China
- 81872601/National Natural Science Foundation of China
- 2022A0505050035/Science and Technology Project of Guangdong Province, China
- 2022A1515010610/Guangdong Basic Applied Basic Research Foundation
- 2022A1515111098/Guangdong-Dongguan Joint grant
- 2023A1515110373/Guangdong-Guangzhou Joint grant
- 2023M741553/Chinese Postdoctoral Science Foundation
- 2023T160295/Chinese Postdoctoral Science Foundation
- 2022M721486/Chinese Postdoctoral Science Foundation
- GZC20231055/Postdoctoral Fellowship Program of CPSF
- 20231800905372/Dongguan Social Development Science and Technology Project-High Level Hospital Construction Project
- 2017B030314035/Guangdong Provincial Key Laboratory of Tropical Disease Research
- 2024ZDZ09/NMPA Key Laboratory for Safety Evaluation of Cosmetics and the GDMPA Project of Scientific and Technological Innovation
LinkOut - more resources
Full Text Sources
