A Randomized Clinical Trial to Evaluate the Efficacy of an Oral Probiotic in Acne Vulgaris
- PMID: 38751177
- PMCID: PMC11110809
- DOI: 10.2340/actadv.v104.33206
A Randomized Clinical Trial to Evaluate the Efficacy of an Oral Probiotic in Acne Vulgaris
Abstract
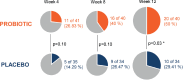
The relevance of the gut microbiota in some skin inflammatory diseases, including acne vulgaris, has been emphasized. Probiotics could play a role in the modulation of the microbiota, improving the clinical course of this disease. A 12-week randomized, double-blind, placebo-controlled, clinical trial with patients aged 12 to 30 years with acne vulgaris was conducted. The study product was a capsule composed of the probiotic Lacticaseibacillus rhamnosus (CECT 30031) and the cyanobacterium Arthrospira platensis (BEA_IDA_0074B). Patients with improvement in the Acne Global Severity Scale were 10/34 (29.41%) in the placebo group compared with 20/40 (50%) in the probiotic group (p = 0.03). A significant reduction (p = 0.03) in the number of non-inflammatory acne lesions was observed in the probiotic group (-18.60 [-24.38 to -12.82]) vs the placebo group (-10.54 [-17.43 to -3.66]). Regarding the number of total lesions, a reduction almost reaching statistical significance (p = 0.06) was observed in the probiotic group (-27.94 [-36.35 to -19.53]) compared with the placebo group (-18.31 [-28.21 to -8.41]). In addition, patients with improvement attending the Global Acne Grading System were 7/34 (20.58%) in the placebo group vs 17/40 (42.50%) in the probiotic group (p = 0.02). The number of adverse events was similar in both groups. The probiotic used in this study was effective and well tolerated, and it should be considered for acne vulgaris patients.
Conflict of interest statement
EN-D, PS-P and VN-L are employees of Bioithas (a sister company of Bionou Research) and VN-L owns stock/stock options.
Figures
References
-
- Bhate K, Williams HC. Epidemiology of acne vulgaris. Br J Dermatol 2013; 168: 474–485. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

