Dorsal root ganglion magnetic resonance imaging biomarker correlations with pain in Fabry disease
- PMID: 38751382
- PMCID: PMC11095551
- DOI: 10.1093/braincomms/fcae155
Dorsal root ganglion magnetic resonance imaging biomarker correlations with pain in Fabry disease
Abstract
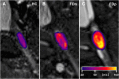
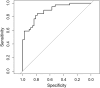
Fabry disease is a rare monogenetic, X-linked lysosomal storage disorder with neuropathic pain as one characteristic symptom. Impairment of the enzyme alpha-galactosidase A leads to an accumulation of globotriaosylceramide in the dorsal root ganglia. Here, we investigate novel dorsal root ganglia MR imaging biomarkers and their association with Fabry genotype and pain phenotype. In this prospective study, 89 Fabry patients were examined using a standardized 3 T MRI protocol of the dorsal root ganglia. Fabry pain was assessed through a validated Fabry pain questionnaire. The genotype was determined by diagnostic sequencing of the alpha-galactosidase A gene. MR imaging end-points were dorsal root ganglia volume by voxel-wise morphometric analysis and dorsal root ganglia T2 signal. Reference groups included 55 healthy subjects and Fabry patients of different genotype categories without Fabry pain. In patients with Fabry pain, T2 signal of the dorsal root ganglia was increased by +39.2% compared to healthy controls (P = 0.001) and by +29.4% compared to painless Fabry disease (P = 0.017). This effect was pronounced in hemizygous males (+40.7% compared to healthy; P = 0.008 and +29.1% compared to painless; P = 0.032) and was consistently observed across the genotype spectrum of nonsense (+38.1% compared to healthy, P < 0.001) and missense mutations (+39.2% compared to healthy; P = 0.009). T2 signal of dorsal root ganglia and globotriaosylsphingosine levels were the only independent predictors of Fabry pain (P = 0.047; P = 0.002). Volume of dorsal root ganglia was enlarged by +46.0% in Fabry males in the nonsense compared to missense genotype category (P = 0.005) and by +34.5% compared to healthy controls (P = 0.034). In painful Fabry disease, MRI T2 signal of dorsal root ganglia is increased across different genotypes. Dorsal root ganglion MRI T2 signal as a novel in vivo imaging biomarker may help to better understand whether Fabry pain is modulated or even caused by dorsal root ganglion pathology.
Keywords: magnetic resonance gangliography; magnetic resonance imaging; magnetic resonance neurography; neuropathic pain; peripheral neuropathy.
© The Author(s) 2024. Published by Oxford University Press on behalf of the Guarantors of Brain.
Conflict of interest statement
P.N. has received speaker and consulting honoraria from Amicus, Chiesi, Idorsia, Sanofi Genzyme and Takeda. C.W. has received honoraria for steering committee membership, advisory boards and lecturing from Amicus, Chiesi, Idorsia, Sanofi and Takeda. C.S. has served on scientific advisory boards for Akcea, Algiax, Air Liquide, Bayer, Grifols, Ipsen, LFB, Immunic, Merz, Pfizer, Roche and Takeda. She has received speaker honoraria from Akcea, Alnylam Amicus, Grifols, Pfizer and Teva. She serves or has served as a journal editor, associate editor or editorial advisory board member for the European Journal of Neurology, PLoS One and PAIN Reports. M.P. has received speaker honoraria from Merck and Bayer. The other authors report no competing interests.
Figures




References
-
- Bangari DS, Ashe KM, Desnick RJ, et al. α-Galactosidase A knockout mice: Progressive organ pathology resembles the type 2 later-onset phenotype of Fabry disease. Am J Pathol. 2015;185(3):651–665. - PubMed
-
- Üçeyler N, Ganendiran S, Kramer D, Sommer C. Characterization of pain in Fabry disease. Clin J Pain. 2014;30(10):915–920. - PubMed
LinkOut - more resources
Full Text Sources
