The Accuracy of the S100B Protein Biomarker in the Prognosis of Patients with Acute Spinal Cord Injury
- PMID: 38751401
- PMCID: PMC11093626
- DOI: 10.1055/s-0043-1771323
The Accuracy of the S100B Protein Biomarker in the Prognosis of Patients with Acute Spinal Cord Injury
Abstract
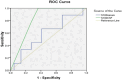
Introduction The role of some biomarkers such as S100 beta (S100B) has been somewhat known in determining the severity of primary acute spinal cord injury (SCI), and today, it has been the basis of various relevant studies. Therefore, this study estimates the S100B level in serum and cerebrospinal fluid (CSF) in patients with spinal injuries. Methods This was a descriptive-analytic study. In this study, 31 patients with acute SCI referred to Sari Imam Khomeini Hospital, Iran, were recruited. Patients were divided into two groups of complete and incomplete SCI according to the American Spinal Injury Association (ASIA). The S100B concentrations in serum and CSF levels were compared between the two groups. Result There was only significant positive correlation between S100B CSF concentration and complete SCI based on the ASIA criterion, meaning that in cases of complete SCI the S100B CSF concentration was significantly increased correlation coefficient (CC) (cc = 0.529 and p = 0.002). Based on the results of serum S100B protein concentration, 14.70 ng/dL with a sensitivity of 66.7% and specificity of 55% was determined as cutoff for complete SCI. Also, about the CSF S100B protein level variable, concentration of 342.18 ng/dL with 100% sensitivity and 64% specificity was determined as cutoff for complete injury. Conclusion The results of this unique study have shown that S100B were useful markers for predicting the prognosis of patients with acute SCI and cutoff points determined for serum and especially CSF concentrations can differentiate complete and incomplete SCI.
Keywords: S100 beta protein; cerebrospinal fluid; spinal cord injury.
Asian Congress of Neurological Surgeons. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. ( https://creativecommons.org/licenses/by-nc-nd/4.0/ ).
Conflict of interest statement
Conflict of Interest None declared.
Figures
References
-
- van den Berg M E, Castellote J M, Mahillo-Fernandez I, de Pedro-Cuesta J.Incidence of spinal cord injury worldwide: a systematic review Neuroepidemiology 20103403184–192., discussion 192 - PubMed
-
- Rodrigues L F, Moura-Neto V. Biomarkers in spinal cord injury: from prognosis to treatment. Mol Neurobiol. 2018;55(08):6436–6448. - PubMed
-
- WHO Spinal cord injuryNovember 19, 2013.https://www.who.int/news-room/fact-sheets/detail/spinal-cord-injury
-
- Rainey T, Lesko M, Sacho R, Lecky F, Childs C. Predicting outcome after severe traumatic brain injury using the serum S100B biomarker: results using a single (24h) time-point. Resuscitation. 2009;80(03):341–345. - PubMed
-
- Fakharian E, Tabesh H, Masoud S A. An epidemiologic study on spinal injury in Kashan. Gilan Univ J. 2006;13(49):79–85.
LinkOut - more resources
Full Text Sources
Miscellaneous