Efficacy and safety of abemaciclib-based therapy versus tucidinostat-based therapy after progression on palbociclib in patients with HR+HER2- metastatic breast cancer
- PMID: 38751483
- PMCID: PMC11093013
- DOI: 10.21037/tbcr-23-9
Efficacy and safety of abemaciclib-based therapy versus tucidinostat-based therapy after progression on palbociclib in patients with HR+HER2- metastatic breast cancer
Abstract
Background: For patients with hormone receptor-positive HER2-negeative metastatic breast cancer (HR+HER2- MBC), switching to another cyclin-dependent kinase 4/6 inhibitor (CDK4/6i) or targeted drugs with different mechanism are considerable treatment strategies post-CDK4/6i. However, no clinical data has been reported on which of the two strategies is more effective. In order to explore optimal treatment option post-CDK4/6i, we performed a retrospective comparative cohort study to evaluate the efficacy and safety of abemaciclib-based therapy versus tucidinostat-based therapy after progression on palbociclib.
Methods: We identified patients with HR+HER2- MBC who had received abemaciclib-based therapy or tucidinostat-based therapy after progression on palbociclib from the database of Chinese Society of Clinical Oncology Breast Cancer (CSCO BC). Baseline characteristics, efficacy and safety information of treatments were derived from seven research centers' medical records. The primary endpoint was progression-free survival (PFS), the secondary endpoints were clinical benefit rate (CBR), PFS according to PIK3CA gene type, and safety.
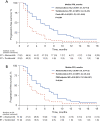
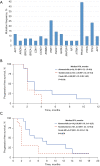
Results: Between April 1st 2020 and June 30th 2022, a total of 149 patients were included, of whom 73 patients received abemaciclib plus endocrine therapy (ET), and 76 patients received tucidinostat plus ET. The majority of patients had visceral disease (124/149, 83.2%) and ≥3 metastatic organs (76/149, 51.0%), one third (48/149, 32.2%) had previously been treated ≥3 lines of ET at baseline in MBC setting. CBR was 38.4% (28/73) in abemaciclib group and 17.1% (13/76) in tucidinostat group (P=0.004). There was significant difference in PFS between abemaciclib group and tucidinostat group in both the whole population (5.0 vs. 2.0 months; hazard ratio =0.44; 95% CI: 0.31-0.64; P<0.001) and propensity score matched population. PIK3CA mutations occurred in 44.20% of patients who had undergone multigene sequencing. PIK3CA-mutant showed a negative effect on PFS of abemaciclib-based therapy. Neutropenia was the most common adverse event in both groups for any grade and grades 3-4. Common non-hematological toxicity occurred in abemaciclib group was diarrhea (27.4%), and were increased aspartate aminotransferase (AST) (26.3%), nausea (25.0%), vomiting (11.8%) and hypokalemia (13.2%) in tucidinostat group.
Conclusions: Our study suggests superiority of abemaciclib-based therapy over tucidinostat-based therapy in patients progressed on palbociclib, which merits further assessment in larger and prospective trials.
Keywords: CDK4/6 inhibitor; abemaciclib; hormone receptor-positive metastatic breast cancer (HR+ MBC); progression; tucidinostat.
2023 Translational Breast Cancer Research. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tbcr.amegroups.org/article/view/10.21037/tbcr-23-9/coif). YW is a full-time employee of Medical Department, Medpion (Beijing) Medical Technology Co., Ltd., Beijing and LZ is a full-time employee of Genecast (Beijing) Biotechnology Co., Ltd. Yongmei Yin, SW, and TS serve as unpaid editorial board members of Translational Breast Cancer Research from March 2022 to February 2024. JL serves as the unpaid managing editor of Translational Breast Cancer Research from November 2019 to October 2024. ZJ serves as the Editor-in-Chief of Translational Breast Cancer Research. The authors have no other conflicts of interest to declare.
Figures

Similar articles
-
Abemaciclib plus endocrine therapy versus chemotherapy after progression on prior palbociclib in HR+/HER2- metastatic breast cancer: A single center real-world study in China.Cancer Med. 2024 May;13(10):e7249. doi: 10.1002/cam4.7249. Cancer Med. 2024. PMID: 38770648 Free PMC article.
-
Clinical outcomes of tucidinostat-based therapy after prior CDK4/6 inhibitor progression in hormone receptor-positive heavily pretreated metastatic breast cancer.Breast. 2022 Dec;66:255-261. doi: 10.1016/j.breast.2022.10.018. Epub 2022 Nov 2. Breast. 2022. PMID: 36375386 Free PMC article.
-
Real-world comparison of palbociclib, abemaciclib, and dalpiciclib as first-line treatments for Chinese HR+/HER2-metastatic breast cancer patients: a multicenter study (YOUNGBC-28).Ther Adv Med Oncol. 2024 Dec 17;16:17588359241302018. doi: 10.1177/17588359241302018. eCollection 2024. Ther Adv Med Oncol. 2024. PMID: 39697620 Free PMC article.
-
CDK4/6 Inhibitors Expand the Therapeutic Options in Breast Cancer: Palbociclib, Ribociclib and Abemaciclib.BioDrugs. 2019 Apr;33(2):125-135. doi: 10.1007/s40259-019-00337-6. BioDrugs. 2019. PMID: 30847853 Review.
-
Association of Cyclin-Dependent Kinases 4 and 6 Inhibitors With Survival in Patients With Hormone Receptor-Positive Metastatic Breast Cancer: A Systematic Review and Meta-analysis.JAMA Netw Open. 2020 Oct 1;3(10):e2020312. doi: 10.1001/jamanetworkopen.2020.20312. JAMA Netw Open. 2020. PMID: 33048129 Free PMC article.
Cited by
-
Impact of HER2-targeting antibody drug conjugates in treatment strategies for patients with breast cancer.Heliyon. 2025 Jan 3;11(3):e41590. doi: 10.1016/j.heliyon.2024.e41590. eCollection 2025 Feb 15. Heliyon. 2025. PMID: 39916839 Free PMC article. Review.
-
Efficacy and safety of tucidinostat in patients with advanced hormone receptor-positive human epidermal growth factor receptor 2-negative breast cancer: real-world insights.Ann Transl Med. 2023 Dec 20;11(12):409. doi: 10.21037/atm-23-1913. Epub 2023 Dec 19. Ann Transl Med. 2023. PMID: 38213803 Free PMC article.
-
Navigating next-generation HR+/HER2- metastatic breast cancer therapies: a critical commentary on abemaciclib vs. tucidinostat after palbociclib progression.Transl Breast Cancer Res. 2023 Oct 23;4:31. doi: 10.21037/tbcr-23-41. eCollection 2023. Transl Breast Cancer Res. 2023. PMID: 38751459 Free PMC article. No abstract available.
-
Abemaciclib-based therapy versus tucidinostat-based therapy in patients with HR+HER2- metastatic breast cancer after palbociclib progression: insights and challenges from a comparative cohort study in China.Transl Breast Cancer Res. 2023 Oct 23;4:32. doi: 10.21037/tbcr-23-45. eCollection 2023. Transl Breast Cancer Res. 2023. PMID: 38751484 Free PMC article. No abstract available.
-
Chinese Society of Clinical Oncology Breast Cancer (CSCO BC) Guidelines in 2024: International Contributions from China.Cancer Biol Med. 2024 Oct 21;21(10):838-43. doi: 10.20892/j.issn.2095-3941.2024.0374. Cancer Biol Med. 2024. PMID: 39434380 Free PMC article. No abstract available.
References
-
- NCCN Clinical Practice Guidelines in Oncology—Breast Cancer Version 1.2022. Accessed Nov 24, 2021. Available online: https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
