Primary results of ELAINA: a randomized, multicenter, open-label, phase III study of the efficacy and safety of trastuzumab emtansine vs. lapatinib plus capecitabine in Chinese patients with HER2-positive locally advanced or metastatic breast cancer who have received prior trastuzumab-based therapy
- PMID: 38751488
- PMCID: PMC11093095
- DOI: 10.21037/tbcr-23-2
Primary results of ELAINA: a randomized, multicenter, open-label, phase III study of the efficacy and safety of trastuzumab emtansine vs. lapatinib plus capecitabine in Chinese patients with HER2-positive locally advanced or metastatic breast cancer who have received prior trastuzumab-based therapy
Abstract
Background: The antibody-drug conjugate (ADCs) trastuzumab emtansine (T-DM1) is approved for human epidermal growth factor receptor 2 (HER2)-positive metastatic breast cancer (mBC) previously treated with trastuzumab and a taxane. The phase III ELAINA trial aimed to determine the clinical utility of T-DM1 in Chinese patients.
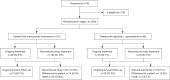
Methods: ELAINA was a randomized, multicenter, open-label bridging study of Chinese patients with HER2-positive locally advanced breast cancer (LABC) or mBC previously treated with trastuzumab and a taxane. Using an interactive voice/internet response system, patients were randomized 3:1 to receive T-DM1 or lapatinib plus capecitabine. Patents were stratified by number of prior therapies in this disease setting and by presence of visceral disease using a permuted block randomization scheme. Patients received treatment until disease progression, unmanageable toxicity, or study termination. After that, data on survival and subsequent cancer therapies were collected at approximately 3-month intervals. The primary endpoint was investigator-assessed progression-free survival (PFS). Secondary endpoints were overall response rate, duration of response, overall survival (OS), safety, patient-reported quality of life, and pharmacokinetics (PKs).
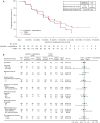
Results: ELAINA was fully enrolled with 200 patients randomized to T-DM1 (n=151) or lapatinib plus capecitabine (n=49). Median treatment duration was approximately 6 months in each study arm. Median follow-up time was approximately 9 months for all analyses except for OS. T-DM1 was associated with a 15% reduction in risk of disease progression or death compared with lapatinib plus capecitabine [stratified hazard ratio (HR) =0.85; 95% confidence interval (CI): 0.56-1.29] in the intent-to-treat (ITT) population. The objective response rate (ORR) was similar with T-DM1 (50.4%) and lapatinib plus capecitabine (55.8%); median duration of response was 8.4 months for both treatments. At a median follow-up time of approximately 30 months, OS was similar in each treatment arm. Incidence of grade ≥3 adverse events (AEs) was similar with T-DM1 (54.3%) and lapatinib plus capecitabine (57.1%). Grade ≥3 thrombocytopenia was greater with T-DM1 (40.4%) than with lapatinib plus capecitabine (4.1%); there was no grade ≥3 hemorrhage with either treatment.
Conclusions: T-DM1 demonstrated an acceptable benefit-risk profile in Chinese patients with HER2-positive LABC/mBC previously treated with trastuzumab and a taxane. T-DM1 therefore provides a chemotherapy-free option in this setting.
Trial registration: ClinicalTrials.gov identifier: NCT03084939.
Keywords: ELAINA bridging study; human epidermal growth factor receptor 2-positive (HER2-positive) breast cancer; metastatic breast cancer (mBC); trastuzumab emtansine (T-DM1).
2023 Translational Breast Cancer Research. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tbcr.amegroups.com/article/view/10.21037/tbcr-23-2/coif). FW, FL, and ER are employees of and hold stock in F. Hoffmann-La Roche. XW serves as an unpaid editorial board member of Translational Breast Cancer Research from December 2022 to November 2024. QZ, YY serves as unpaid editorial board members of Translational Breast Cancer Research from March 2022 to February 2024. ZJ serves as the Editor-in-Chief of Translational Breast Cancer Research. The other authors have no conflicts of interest to declare.
Figures

Similar articles
-
Trastuzumab emtansine versus capecitabine plus lapatinib in patients with previously treated HER2-positive advanced breast cancer (EMILIA): a descriptive analysis of final overall survival results from a randomised, open-label, phase 3 trial.Lancet Oncol. 2017 Jun;18(6):732-742. doi: 10.1016/S1470-2045(17)30312-1. Epub 2017 May 16. Lancet Oncol. 2017. PMID: 28526536 Free PMC article. Clinical Trial.
-
Efficacy and Safety of Trastuzumab Emtansine Plus Capecitabine vs Trastuzumab Emtansine Alone in Patients With Previously Treated ERBB2 (HER2)-Positive Metastatic Breast Cancer: A Phase 1 and Randomized Phase 2 Trial.JAMA Oncol. 2020 Aug 1;6(8):1203-1209. doi: 10.1001/jamaoncol.2020.1796. JAMA Oncol. 2020. PMID: 32584367 Free PMC article. Clinical Trial.
-
Trastuzumab emtansine for HER2-positive advanced breast cancer.N Engl J Med. 2012 Nov 8;367(19):1783-91. doi: 10.1056/NEJMoa1209124. Epub 2012 Oct 1. N Engl J Med. 2012. PMID: 23020162 Free PMC article. Clinical Trial.
-
Trastuzumab Emtansine for Treating HER2-Positive, Unresectable, Locally Advanced or Metastatic Breast Cancer After Treatment with Trastuzumab and a Taxane: An Evidence Review Group Perspective of a NICE Single Technology Appraisal.Pharmacoeconomics. 2016 Jul;34(7):673-80. doi: 10.1007/s40273-016-0386-z. Pharmacoeconomics. 2016. PMID: 26892972 Review.
-
Ado-trastuzumab emtansine: a HER2-positive targeted antibody-drug conjugate.Ann Pharmacother. 2014 Nov;48(11):1484-93. doi: 10.1177/1060028014545354. Epub 2014 Jul 31. Ann Pharmacother. 2014. PMID: 25082874 Review.
Cited by
-
Real-world treatment patterns and outcomes among patients with HER2-positive unresectable or metastatic breast cancer in China.Front Oncol. 2025 May 14;15:1527990. doi: 10.3389/fonc.2025.1527990. eCollection 2025. Front Oncol. 2025. PMID: 40438675 Free PMC article.
-
Inetetamab combined with pyrotinib and oral vinorelbine for patients with human epidermal growth factor receptor 2 positive advanced breast cancer: A single-arm phase 2 clinical trial.Cancer Pathog Ther. 2023 Oct 30;2(1):31-37. doi: 10.1016/j.cpt.2023.10.004. eCollection 2024 Jan. Cancer Pathog Ther. 2023. PMID: 38328709 Free PMC article.
References
-
- NCCN Clinical Practice Guidelines in Oncology – Breast cancer, version 2.2022. December 20, 2021.
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
