Statement on the toxicological properties and maximum residue levels of acetamiprid and its metabolites
- PMID: 38751503
- PMCID: PMC11094581
- DOI: 10.2903/j.efsa.2024.8759
Statement on the toxicological properties and maximum residue levels of acetamiprid and its metabolites
Abstract
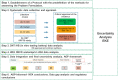
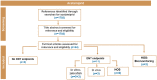
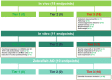
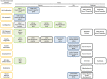
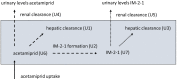
Acetamiprid is a pesticide active substance with insecticidal action whose approval was renewed by Commission Implementing Regulation (EU) 2018/113. In January 2022, the EFSA PPR Panel published a statement following a request from the European Commission to advise on human health or the environment based on new scientific evidence presented by France during the decision-making phase. In July 2022, by means of a further mandate received from the European Commission, EFSA was requested to provide advice if new information and any other scientific evidence that has become available since the assessment conducted for the renewal in 2018 warrant re-evaluation of (i) toxicological parameters used for the risk assessment of acetamiprid during the renewal process, including toxicological endpoints; (ii) the residue definition for acetamiprid in products of plant origin; and (iii) the safety of existing maximum residue levels (MRLs). Meanwhile, the applicant of acetamiprid in the EU submitted new toxicology studies regarding the toxicological profile of the metabolite IM-2-1. Furthermore, the European Commission was made aware that several recent publications in scientific literature were made available after the literature searches conducted by EFSA. As the new data could affect the advice that EFSA was expected to deliver through the 2022 mandate, EFSA was further requested to consider this information by means of a revised mandate received in September 2023. As regards re-evaluation of point (i) in this statement, this was addressed by an EFSA Working Group integrating all the available evidence. The results of the weight of evidence indicated that there are major uncertainties in the body of evidence for the developmental neurotoxicity (DNT) properties of acetamiprid and further data are therefore needed to come to a more robust mechanistic understanding to enable appropriate hazard and risk assessment. In view of these uncertainties, the EFSA WG proposed to lower the acceptable daily intake (ADI) and acute reference dose (ARfD) from 0.025 to 0.005 mg/kg body weight (per day). A revised residue definition for risk assessment was proposed for leafy and fruit crops as sum of acetamiprid and N-desmethyl-acetamiprid (IM-2-1), expressed as acetamiprid. Regarding pulses/oilseeds, root crops and cereals, the new data received did not indicate a need to modify the existing residue definition for risk assessment, which therefore remains as parent acetamiprid. Regarding the residue definition for enforcement, the available data did not indicate a need to modify the existing definition because acetamiprid is still a sufficient marker of the residues in all crop groups. Considering the new health-based guidance values derived in the present statement, a risk for consumer has been identified for 38 MRLs currently in place in the EU Regulation. Consequently, EFSA recommended to lower the existing MRLs for 38 commodities based on the assessment of fall-back Good Agricultural Practices received within an ad hoc data call. Some fall-back MRLs proposals require further risk management considerations.
Keywords: acetamiprid; developmental neurotoxicity; insecticides; maximum residue levels; metabolite IM‐2‐1; monitoring data.
© 2024 European Food Safety Authority. EFSA Journal published by Wiley‐VCH GmbH on behalf of European Food Safety Authority.
Conflict of interest statement
If you wish to access the declaration of interests of any expert contributing to an EFSA scientific assessment, please contact interestmanagement@efsa.europa.eu.
Figures


Similar articles
-
Focussed assessment of certain existing MRLs of concern for acetamiprid and modification of the existing MRLs for table olives, olives for oil production, barley and oats.EFSA J. 2018 May 16;16(5):e05262. doi: 10.2903/j.efsa.2018.5262. eCollection 2018 May. EFSA J. 2018. PMID: 32625899 Free PMC article.
-
Targeted review of maximum residues levels (MRLs) for indoxacarb.EFSA J. 2022 Aug 9;20(8):e07527. doi: 10.2903/j.efsa.2022.7527. eCollection 2022 Aug. EFSA J. 2022. PMID: 35958103 Free PMC article.
-
Modification of the existing maximum residue levels for acetamiprid in honey and various oilseed crops.EFSA J. 2022 Aug 24;20(8):e07535. doi: 10.2903/j.efsa.2022.7535. eCollection 2022 Aug. EFSA J. 2022. PMID: 36034320 Free PMC article.
-
Safety and nutritional assessment of GM plants and derived food and feed: the role of animal feeding trials.Food Chem Toxicol. 2008 Mar;46 Suppl 1:S2-70. doi: 10.1016/j.fct.2008.02.008. Epub 2008 Feb 13. Food Chem Toxicol. 2008. PMID: 18328408 Review.
-
Translating data into policy informing decisions: current and future perspectives from the European Food Safety Authority (EFSA).Proc Nutr Soc. 2025 Feb 5:1-9. doi: 10.1017/S0029665125000047. Online ahead of print. Proc Nutr Soc. 2025. PMID: 39905747 Review.
Cited by
-
Laboratory and field efficacy of natural products against the invasive pest Halyomorpha halys and side effects on the biocontrol agent Trissolcus japonicus.Sci Rep. 2025 Feb 7;15(1):4622. doi: 10.1038/s41598-025-87325-9. Sci Rep. 2025. PMID: 39920209 Free PMC article.
-
Target-driven ratiometric electrochemical aptasensor based on polymerization and AuNP signal amplification for acetamiprid residue determination.Mikrochim Acta. 2025 Jun 23;192(7):443. doi: 10.1007/s00604-025-07217-7. Mikrochim Acta. 2025. PMID: 40549055
-
Scientific support for preparing an EU position in the 55th Session of the Codex Committee on Pesticide Residues (CCPR).EFSA J. 2024 Jul 18;22(7):e8841. doi: 10.2903/j.efsa.2024.8841. eCollection 2024 Jul. EFSA J. 2024. PMID: 39026987 Free PMC article.
-
The 2023 European Union report on pesticide residues in food.EFSA J. 2025 May 14;23(5):e9398. doi: 10.2903/j.efsa.2025.9398. eCollection 2025 May. EFSA J. 2025. PMID: 40371318 Free PMC article.
-
The impact of neonicotinoid pesticides on reproductive health.Toxicol Sci. 2025 Feb 1;203(2):131-146. doi: 10.1093/toxsci/kfae138. Toxicol Sci. 2025. PMID: 39460954 Review.
References
-
- Austria . (2023). Evaluation report prepared under Article 31 of Regulation (EC) No 178/2002. October 2023. Additional data to be considered for the assessment of maximum residue levels (MRLs) of acetamiprid.
-
- Belgium . (2023). Evaluation report prepared under Article 31 of Regulation (EC) No 178/2002. November 2023. Additional data to be considered for the assessment of maximum residue levels (MRLs) of acetamiprid.
-
- Blum, J. , Masjosthusmann, S. , Bartmann, K. , Bendt, F. , Dolde, X. , Dönmez, A. , Förster, N. , Holzer, A. K. , Hübenthal, U. , Keßel, H. E. , Kilic, S. , Klose, J. , Pahl, M. , Stürzl, L. C. , Mangas, I. , Terron, A. , Crofton, K. M. , Scholze, M. , Mosig, A. , … Fritsche, E. (2023). Establishment of a human cell‐based in vitro battery to assess developmental neurotoxicity hazard of chemicals. Chemosphere, 311(Pt 2), 137035. 10.1016/j.chemosphere.2022.137035 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
