Sequential versus concurrent adjuvant chemo-endocrine therapy for HR+ early breast cancer: a systematic review and Bayesian network meta-analysis
- PMID: 38751511
- PMCID: PMC11093067
- DOI: 10.21037/tbcr-21-3
Sequential versus concurrent adjuvant chemo-endocrine therapy for HR+ early breast cancer: a systematic review and Bayesian network meta-analysis
Abstract
Background: Chemo-endocrine therapy is the standard adjuvant treatment strategy for hormone receptor-positive (HR+) early breast cancer. Our research aimed to compare the efficacy of adjuvant chemo-endocrine therapies, regarding different endocrinal regimens and integration sequences (sequential or concomitant), for HR+ early breast cancer.
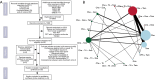
Methods: PubMed, Embase, the Cochrane Library and web of science were searched for articles published before October 2018 with Clinicaltrials.gov (https://clinicaltrials.gov) for registered clinical trials and ASCO, AACR, ESCO, SABCS meeting abstracts for addition. Randomized clinical trials (RCTs) comparing chemotherapy and/or endocrine therapy in the adjuvant treatment of primary breast cancer patients were included. Hazard ratios (HRs) of disease-free survival (DFS) and overall survival (OS) were extracted and analyzed in Bayesian analysis. Patients were stratified by menopause status.
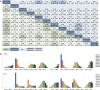
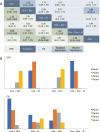
Results: Thirty-three trials with 28,515 patients and 19 treatments were enrolled. Comparisons between regimens has seen better efficacy of ovarian function suppressor (OFS) + aromatase inhibitors (AI) than OFS + tamoxifen, either used concurrently [HR =0.69, 95% credible intervals (CrI): 0.47-1.02] or sequentially with chemotherapy (HR =0.72, 95% CrI: 0.49-1.06) in premenopausal patients. Adding OFS to tamoxifen was marginally better than tamoxifen used alone (DFS: HR =0.85, 95% CrI: 0.65-1.09; OS: HR =0.77, 95% CrI: 0.52-1.08). Comparisons between different sequences of chemo-endocrine therapy proved equal efficacy in premenopausal and postmenopausal patients. Recommendation was given based on ranking of treatments. Sequential and concurrent use of chemotherapy and OFS + AI ranked equally in premenopausal patients and were recommended as the best option. However, tamoxifen ranked higher when used concurrently with chemotherapy in both premenopausal and postmenopausal HR+ early breast cancer.
Conclusions: In the adjuvant chemo-endocrine therapy for premenopausal HR+ early breast cancer, concurrent and sequential adjuvant chemo-endocrine therapy was demonstrated of equal efficacy in both postmenopausal and premenopausal HR+ early breast cancer.
Trial registration: PROSPERO CRD42018104889.
Keywords: Bayesian analysis; Estrogen receptor-positive breast cancer; chemotherapy; endocrine therapy; randomized clinical trial (RCTs).
2022 Translational Breast Cancer Research. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tbcr.amegroups.com/article/view/10.21037/tbcr-21-3/coif). The authors have no conflicts of interest to declare.
Figures
Similar articles
-
Systematic literature review and trial-level meta-analysis of aromatase inhibitors vs tamoxifen in patients with HR+/HER2- early breast cancer.Breast. 2025 Jun;81:104429. doi: 10.1016/j.breast.2025.104429. Epub 2025 Mar 5. Breast. 2025. PMID: 40153939 Free PMC article.
-
Adjuvant ovarian function suppression and tamoxifen in premenopausal breast cancer patients: A meta-analysis.Curr Probl Cancer. 2020 Dec;44(6):100592. doi: 10.1016/j.currproblcancer.2020.100592. Epub 2020 May 26. Curr Probl Cancer. 2020. PMID: 32527567
-
Comparative efficacy and safety of extended adjuvant endocrine therapy for hormone receptor-positive early breast cancer: a Bayesian network meta-analysis.Breast Cancer Res Treat. 2024 Jan;203(1):13-28. doi: 10.1007/s10549-023-07105-9. Epub 2023 Oct 3. Breast Cancer Res Treat. 2024. PMID: 37787817 Review.
-
Aromatase inhibitors plus ovarian function suppression versus tamoxifen plus ovarian function suppression for premenopausal women with early stage breast cancer: a systematic review and meta-analysis.Ann Palliat Med. 2020 Jul;9(4):2294-2302. doi: 10.21037/apm-20-488A. Epub 2020 May 20. Ann Palliat Med. 2020. PMID: 32434371
-
Adjuvant ovarian suppression for premenopausal hormone receptor-positive breast cancer: A network meta-analysis.Medicine (Baltimore). 2021 Aug 20;100(33):e26949. doi: 10.1097/MD.0000000000026949. Medicine (Baltimore). 2021. PMID: 34414958 Free PMC article.
Cited by
-
Optimizing Premenopausal Hormone Receptor-Positive Human Epidermal Growth Factor Receptor 2-Negative Early Breast Cancer Management in India: Insights From Expert Consensus.Cureus. 2024 Dec 25;16(12):e76392. doi: 10.7759/cureus.76392. eCollection 2024 Dec. Cureus. 2024. PMID: 39867062 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
