A narrative review of the clinical development of CDK4/6 inhibitor abemaciclib in breast cancer
- PMID: 38751545
- PMCID: PMC11093002
- DOI: 10.21037/tbcr-21-36
A narrative review of the clinical development of CDK4/6 inhibitor abemaciclib in breast cancer
Abstract
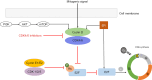
Background and objective: Advanced or metastatic breast cancer (MBC) is associated with poor prognosis and presents many challenges in medical management and treatment decisions. Anticancer drugs that act on cell cycle mechanisms have shown great potential in preclinical studies. In clinical trials, abemaciclib, a reversible ATP-competitive cyclin-dependent kinase 4/6 (CDK4/6) inhibitor developed by Eli Lilly and Company, combined with endocrine therapy (ET) were associated with superior outcomes compared with ET alone in patients with advanced or metastatic hormone receptor positive (HR+)/human epidermal growth factor receptor 2 negative (HER2-) breast cancer, representing a new standard-of-care in this population. Abemaciclib has been approved by the U.S. Food and Drug Administration (FDA) for use in HR+/HER2- MBC. In China, abemaciclib was also approved by the National Medical Products Administration (NMPA) based on findings from the MONARCH plus trial. Recently, abemaciclib have been approved as the first and only CDK4/6 inhibitor by FDA and NMPA for use in HR+/HER2-, node-positive, early breast cancer (EBC) at high risk of recurrence and Ki-67 score ≥20%. Further trials of abemaciclib are ongoing. This is an overview of the clinical development of abemaciclib in breast cancer.
Methods: We reviewed English publications in PubMed related to CDK4/6 inhibitors from 2011 to 2021.
Key content and findings: In this review, we summarized the mechanism, results of preclinical and clinical studies of abemaciclib, describing current indications for treatment, ongoing clinical trials, safety and tolerability, and future perspectives.
Conclusions: Abemaciclib is a unique CDK4/6 inhibitor with distinctive characteristics and promising data, which bring benefit to HR+, HER2- breast cancer patients.
Keywords: Cyclin-dependent kinase 4/6 inhibitor (CDK4/6 inhibitor); abemaciclib; hormone receptor positive/human epidermal growth factor receptor 2 negative breast cancer (HR+/HER2− breast cancer); metastatic breast cancer (MBC).
2022 Translational Breast Cancer Research. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tbcr.amegroups.com/article/view/10.21037/tbcr-21-36/coif). NW is a former employee of Eli Lilly and Company. LY, YC, and WH are current employees of Eli Lilly and Company.
Figures
Similar articles
-
Abemaciclib: a CDK4/6 inhibitor for the treatment of HR+/HER2- advanced breast cancer.Drug Des Devel Ther. 2018 Feb 16;12:321-330. doi: 10.2147/DDDT.S137783. eCollection 2018. Drug Des Devel Ther. 2018. PMID: 29497278 Free PMC article. Review.
-
Abemaciclib: The First FDA-Approved CDK4/6 Inhibitor for the Adjuvant Treatment of HR+ HER2- Early Breast Cancer.Ann Pharmacother. 2022 Feb 8:10600280211073322. doi: 10.1177/10600280211073322. Online ahead of print. Ann Pharmacother. 2022. PMID: 35135362
-
Abemaciclib combined with endocrine therapy as adjuvant treatment for hormone-receptor-positive, HER2-, high-risk early breast cancer: 5-year Chinese population analysis of the phase III randomized monarchE study.Ther Adv Med Oncol. 2024 Oct 23;16:17588359241286775. doi: 10.1177/17588359241286775. eCollection 2024. Ther Adv Med Oncol. 2024. PMID: 39463748 Free PMC article.
-
Abemaciclib: The Newest CDK4/6 Inhibitor for the Treatment of Breast Cancer.Ann Pharmacother. 2019 Feb;53(2):178-185. doi: 10.1177/1060028018795146. Epub 2018 Aug 13. Ann Pharmacother. 2019. PMID: 30099886 Review.
-
Abemaciclib: A Review in Early Breast Cancer with a High Risk of Recurrence.Target Oncol. 2023 Mar;18(2):287-294. doi: 10.1007/s11523-023-00952-y. Epub 2023 Feb 24. Target Oncol. 2023. PMID: 36826463 Review.
Cited by
-
An Overview of the Safety Profile and Clinical Impact of CDK4/6 Inhibitors in Breast Cancer-A Systematic Review of Randomized Phase II and III Clinical Trials.Biomolecules. 2023 Sep 20;13(9):1422. doi: 10.3390/biom13091422. Biomolecules. 2023. PMID: 37759823 Free PMC article.
-
CDK4/6 inhibitors in breast cancer therapy: mechanisms of drug resistance and strategies for treatment.Front Pharmacol. 2025 May 12;16:1549520. doi: 10.3389/fphar.2025.1549520. eCollection 2025. Front Pharmacol. 2025. PMID: 40421216 Free PMC article. Review.
References
-
- Ning Z. Why ACE—overview of the development of the subtype-selective histone deacetylase inhibitor chidamide in hormone receptor positive advanced breast cancer. Transl Breast Cancer Res 2020;1:5. 10.21037/tbcr.2020.03.06 - DOI
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
