Balancing Efficacy and Tolerability of First-Line Systemic Therapies for Advanced Hepatocellular Carcinoma: A Network Meta-Analysis
- PMID: 38751554
- PMCID: PMC11095611
- DOI: 10.1159/000531744
Balancing Efficacy and Tolerability of First-Line Systemic Therapies for Advanced Hepatocellular Carcinoma: A Network Meta-Analysis
Abstract
Background: Atezolizumab + bevacizumab represent the current standard of care for first-line treatment of advanced hepatocellular carcinoma (HCC). However, direct comparison with other combination treatments including immune checkpoint inhibitors (ICI) + tyrosine kinase inhibitors (TKIs) are lacking.
Objectives: This network meta-analysis (NMA) aims to indirectly compare the efficacy and the safety of first-line systemic therapies for unresectable advanced HCC.
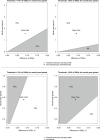
Method: A literature search of MEDLINE, Embase, and SCOPUS databases was conducted up to October 31, 2022. Phase 3 randomized controlled trials (RCTs) testing TKIs, including sorafenib and lenvatinib, or ICIs reporting overall survival (OS) and progression-free survival (PFS) were included. Individual survival data were extracted from OS and PFS curves to calculate restricted mean survival time. A Bayesian NMA was performed to compare treatments in terms of efficacy (15- and 30-month OS, 6-month PFS) and safety, represented by grade ≥3 (severe) adverse events (SAEs). The incremental safety-effectiveness ratio as measure of net health benefit was calculated as the difference in SAE probability divided by survival difference between the 2 most effective treatments.
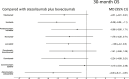
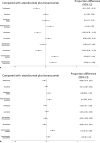
Results: Nine RCTs enrolling 6,600 patients were included. Atezolizumab plus bevacizumab showed the highest probability (88%) of achieving the 30-month OS landmark. Lenvatinib showed a probability of 86% of achieving best PFS outcomes. ICI monotherapies ranked as most tolerable. Atezolizumab plus bevacizumab showed the best net health benefit for OS, compared to durvalumab plus tremelimumab. When evaluating the net health benefit for PFS, at a willingness-to-risk threshold of 10% of SAEs for life-month gained, atezolizumab plus bevacizumab was favoured in 78% of cases, while at threshold of 30% of SAEs for life-month gained, lenvatinib was favoured in 76% of cases.
Conclusions: Atezolizumab plus bevacizumab is the best treatment in terms of net benefit and therefore it should be recommended as standard of care. Compared to atezolizumab plus bevacizumab, lenvatinib monotherapy had the best net benefit for PFS when physicians and patients are available to accept a higher risk of toxicity.
Keywords: Anti-vascular endothelial growth factor; First-line treatment; Hepatocellular carcinoma; Immunotherapy; Systemic treatment; Tyrosin-kinase inhibitors.
© 2023 The Author(s). Published by S. Karger AG, Basel.
Conflict of interest statement
Giuseppe Cabibbo participated in advisory board for Bayer, Eisai, Ipsen, and AstraZeneca. Ciro Celsa received speaker fees from Eisai, Ipsen, and MSD. David James Pinato received lecture fees from BMS, Roche, and EISAI; travel expenses from BMS and Bayer Healthcare; consulting fees from Mina Therapeutics, EISAI, Roche, Da Volterra, Ewopharma, and AstraZeneca; research funding (to the institution) from MSD, GSK, and BMS; grant funding from the Wellcome Trust Strategic Fund (PS3416) and from the Associazione Italiana per la Ricerca sul Cancro (AIRC MFAG Grant ID 25697); and support from the NIHR Imperial Biomedical Research Centre, the Imperial Experimental Cancer Medicine Centre (ECMC), and the Imperial College Tissue Bank. Calogero Cammà participated in the advisory board for Bayer, MSD/Merck, Ipsen, AstraZeneca, Roche, and Eisai. The other authors have no disclosure to declare.
Figures


References
-
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. Erratum in: CA Cancer J Clin. 2020 Jul;70(4):313. - PubMed
-
- Cabibbo G, Enea M, Attanasio M, Bruix J, Craxì A, Cammà C. A meta-analysis of survival rates of untreated patients in randomized clinical trials of hepatocellular carcinoma. Hepatology. 2010 Apr;51(4):1274–83. - PubMed
-
- Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. . Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008 Jul 24;359(4):378–90. - PubMed
-
- Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, et al. . Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018 Mar 24;391(10126):1163–73. - PubMed
LinkOut - more resources
Full Text Sources

