Genetic/Epigenetic Alteration and Tumor Immune Microenvironment in Intrahepatic Cholangiocarcinoma: Transforming the Immune Microenvironment with Molecular-Targeted Agents
- PMID: 38751556
- PMCID: PMC11095601
- DOI: 10.1159/000534443
Genetic/Epigenetic Alteration and Tumor Immune Microenvironment in Intrahepatic Cholangiocarcinoma: Transforming the Immune Microenvironment with Molecular-Targeted Agents
Abstract
Background: Intrahepatic cholangiocarcinoma (iCCA) is often diagnosed at an advanced stage, leading to limited treatment options and a poor prognosis. So far, standard systemic therapy for advanced iCCA has been a combination of gemcitabine and cisplatin. However, recent advancements in the understanding of the molecular characteristics of iCCA have opened new possibilities for molecular-targeted therapies and immunotherapy.
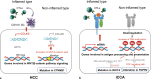
Summary: Reportedly, 9-36% of iCCA cases have an inflamed tumor immune microenvironment (TME) based on the immune gene expression signature, which is characterized by the presence of immune cells involved in anti-tumor immune responses. The majority of iCCA cases have a non-inflamed TME with a lack of effector T cells, rendering immune checkpoint inhibitors (ICIs) ineffective in these cases. Interestingly, alterations in the fibroblast growth factor receptor (FGFR2) gene and IDH1/2 gene mutations are often observed in the non-inflamed TME in iCCA. Several mechanisms have been reported for the role of driver mutations on the establishment of TME unique for iCCA. For example, IDH1/2 mutations, which cause an increase in DNA methylation, are associated with the downregulation and hypermethylation of antigen processing and presentation machinery, which may contribute to the establishment of a non-inflamed TME. Therefore, inhibitors targeting IDH1/2 may restore the DNA methylation and expression status of molecules involved in antigen presentation, potentially improving the efficacy of ICIs. FGFR inhibitors may also have the potential to modulate immunosuppressive TME by inhibitingthe suppressor of cytokine signaling 1 and activating the interferon-γ signaling as a consequence of inhibition of the FGFR signal. From this perspective, understanding the molecular characteristics of iCCA, including the TME and driver mutations, is essential for the effective application of ICIs and molecular-targeted therapies.
Key messages: Combination approaches that target both the tumor and immune system hold promise for improving the outcomes of patients with iCCA. Further research and clinical trials are needed to validate these approaches and optimize the treatment strategies for iCCA.
Keywords: Cholangiocarcinoma; Driver mutation; Immune checkpoint inhibitors; Molecular-targeted agents; Tumor immune microenvironment.
© 2023 The Author(s). Published by S. Karger AG, Basel.
Conflict of interest statement
M.K. has received grants from Taiho Pharmaceuticals, Chugai Pharmaceuticals, Otsuka, Takeda, Sumitomo Dainippon-Sumitomo, Daiichi Sankyo, AbbVie, Astellas Pharma, and Bristol-Myers Squibb. He has also received grants and personal lecture fees from Merck Sharpe and Dohme (MSD), Eisai, and Bayer and is an adviser for MSD, Eisai, Bayer, Bristol-Myers Squibb, Eli Lilly, Chugai, AstraZeneca, and ONO Pharmaceuticals. M.K. is an Editor-in-Chief of Liver Cancer, and N.N. is an Editorial Board member of Liver Cancer.
Figures

Similar articles
-
Novel microenvironment-based classification of intrahepatic cholangiocarcinoma with therapeutic implications.Gut. 2023 Apr;72(4):736-748. doi: 10.1136/gutjnl-2021-326514. Epub 2022 May 18. Gut. 2023. PMID: 35584893 Free PMC article.
-
Case Report: Persistent response to combination therapy of pemigatinib, chemotherapy, and immune checkpoint inhibitor in a patient with advanced intrahepatic cholangiocarcinoma.Front Immunol. 2023 May 24;14:1124482. doi: 10.3389/fimmu.2023.1124482. eCollection 2023. Front Immunol. 2023. PMID: 37292215 Free PMC article.
-
Molecular and Pathological Heterogeneity of Synchronous Small and Large Duct Intrahepatic Cholangiocarcinoma-A Case Series.Curr Oncol. 2025 Apr 27;32(5):255. doi: 10.3390/curroncol32050255. Curr Oncol. 2025. PMID: 40422514 Free PMC article.
-
Targeting FGFR in intrahepatic cholangiocarcinoma [iCCA]: leading the way for precision medicine in biliary tract cancer [BTC]?Expert Opin Investig Drugs. 2021 Apr;30(4):463-477. doi: 10.1080/13543784.2021.1900821. Epub 2021 Apr 11. Expert Opin Investig Drugs. 2021. PMID: 33678096 Review.
-
The Immune-Genomics of Cholangiocarcinoma: A Biological Footprint to Develop Novel Immunotherapies.Cancers (Basel). 2025 Jan 15;17(2):272. doi: 10.3390/cancers17020272. Cancers (Basel). 2025. PMID: 39858054 Free PMC article. Review.
Cited by
-
Biomarkers and Management of Cholangiocarcinoma: Unveiling New Horizons for Precision Therapy.Cancers (Basel). 2025 Apr 6;17(7):1243. doi: 10.3390/cancers17071243. Cancers (Basel). 2025. PMID: 40227772 Free PMC article. Review.
-
Research hotspots and trends in immunotherapy for cholangiocarcinoma: a bibliometric analysis (2014-2023).Front Immunol. 2024 Nov 26;15:1436315. doi: 10.3389/fimmu.2024.1436315. eCollection 2024. Front Immunol. 2024. PMID: 39660136 Free PMC article.
-
Immune microenvironment heterogeneity characterizes biologically distinct KRASmut/SPOPmut and KRASmut/PIK3CAmut mesonephric-like adenocarcinoma subtypes revealed by integrated whole-exome and transcriptomic profiling.Front Immunol. 2025 Jul 16;16:1605227. doi: 10.3389/fimmu.2025.1605227. eCollection 2025. Front Immunol. 2025. PMID: 40740763 Free PMC article.
-
Crosstalk of pyroptosis and cytokine in the tumor microenvironment: from mechanisms to clinical implication.Mol Cancer. 2024 Nov 30;23(1):268. doi: 10.1186/s12943-024-02183-9. Mol Cancer. 2024. PMID: 39614288 Free PMC article. Review.
References
-
- Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, et al. . Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010 Apr 8;362(14):1273–81. - PubMed
-
- Goyal L, Meric-Bernstam F, Hollebecque A, Valle JW, Morizane C, Karasic TB, et al. . Futibatinib for FGFR2-rearranged intrahepatic cholangiocarcinoma. N Engl J Med. 2023 Jan 19;388(3):228–39. - PubMed
-
- Oh D-Y, Ruth He A, Qin S, Chen L-T, Okusaka T, Vogel A, et al. . Durvalumab plus gemcitabine and cisplatin in advanced biliary tract cancer. NEJM Evid. 2022;1(8). - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

