Evaluating the feasibility of delivering a pain management programme for adults living with sickle cell disease
- PMID: 38751559
- PMCID: PMC11092933
- DOI: 10.1177/20494637231202744
Evaluating the feasibility of delivering a pain management programme for adults living with sickle cell disease
Abstract
Background: Pain is the prominent feature of sickle cell disease (SCD) and negatively affects quality of life. Delivery of pain management programmes (PMPs) has been suggested in clinical guidelines for pain management in SCD; however, further evidence of the feasibility and effectiveness of PMPs in this population is needed. This study explored the feasibility of delivering a sickle cell pain management programme (SCPMP) for adults within a haemoglobinopathies service.
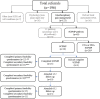
Methods: A single arm, repeated-measures observational design was used to determine feasibility of delivering the SCPMP at one study site. Primary feasibility outcomes were recruitment, completion of treatment and outcome measures, satisfaction, credibility and acceptability to participants. Secondary feasibility outcomes were treatment outcomes and processes, frequency of vaso-occlusive crisis (VOC) and healthcare utilisation.
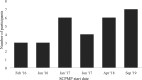
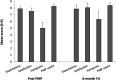
Results: Four of five feasibility criteria were met. Annual recruitment of eight participants to a SCPMP was not achieved. Twenty-nine people began a SCPMP during the study period. Twenty-five (86.2%) participants attended ≥5/8 sessions and 21(84%) programme completers provided all end of programme questionnaires. Mean scores of >7 on ten-point scales were seen across satisfaction and credibility questions. At least moderate (Hedges g >0.5) effect sizes were seen in pre-post SCPMP measures of pain interference, anxiety, depression, self-efficacy, pain-related worry and acceptance. A small (Hedges g 0.4) effect size was seen in HRQoL. Following SCPMP attendance, mean frequency of self-reported VOC and hospital admissions reduced.
Conclusions: This study suggests that, given an adequate source of referrals, a SCPMP is feasible to deliver and appears acceptable and credible to participants. Exploration of influences on recruitment, such as barriers to group interventions, would be illuminating, prior to investigating feasibility of an adequately powered randomised-controlled trial.
Keywords: chronic pain; pain management programme; persistent pain; sickle cell; sickle cell disease.
© The Author(s) 2023.
Conflict of interest statement
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures
Similar articles
-
Pharmacological interventions for painful sickle cell vaso-occlusive crises in adults.Cochrane Database Syst Rev. 2019 Nov 14;2019(11):CD012187. doi: 10.1002/14651858.CD012187.pub2. Cochrane Database Syst Rev. 2019. PMID: 31742673 Free PMC article.
-
Acceptance and commitment therapy for older people with treatment-resistant generalised anxiety disorder: the FACTOID feasibility study.Health Technol Assess. 2021 Sep;25(54):1-150. doi: 10.3310/hta25540. Health Technol Assess. 2021. PMID: 34542399 Clinical Trial.
-
Guided self-help for depression in autistic adults: the ADEPT feasibility RCT.Health Technol Assess. 2019 Dec;23(68):1-94. doi: 10.3310/hta23680. Health Technol Assess. 2019. PMID: 31856942 Free PMC article. Clinical Trial.
-
Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.Cochrane Database Syst Rev. 2022 Feb 1;2(2022):CD014217. doi: 10.1002/14651858.CD014217. Cochrane Database Syst Rev. 2022. PMID: 36321557 Free PMC article.
-
Impact of summer programmes on the outcomes of disadvantaged or 'at risk' young people: A systematic review.Campbell Syst Rev. 2024 Jun 13;20(2):e1406. doi: 10.1002/cl2.1406. eCollection 2024 Jun. Campbell Syst Rev. 2024. PMID: 38873396 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Miscellaneous
