Real-World Patterns and Economic Burden Associated With Treatment Failure With Advanced Therapies in Patients With Moderate-to-Severe Ulcerative Colitis
- PMID: 38751576
- PMCID: PMC11094759
- DOI: 10.1093/crocol/otae026
Real-World Patterns and Economic Burden Associated With Treatment Failure With Advanced Therapies in Patients With Moderate-to-Severe Ulcerative Colitis
Abstract
Background: Some patients lose response during treatment for moderate-to-severe ulcerative colitis (UC). We aimed to characterize real-world treatment failure patterns and associated economic burdens during use of first-line advanced therapies for UC.
Methods: IBM MarketScan Commercial and Medicare Supplemental Databases were used to identify adults initiating ≥ 1 advanced therapy for UC (January 1, 2010-September 30, 2019). Treatment failure was defined as augmentation with non-advanced therapy, discontinuation, dose escalation/interval shortening, failure to taper corticosteroids, UC-related surgery, or UC-related urgent care ≤ 12 months after treatment initiation. The index date was the date of treatment failure (treatment failure cohort) or 12 months after treatment initiation (persistent cohort). Treatment failure rates were assessed using Kaplan-Meier analyses. All-cause and UC-related healthcare resource utilization (HCRU) and costs 12 months post-index were also assessed.
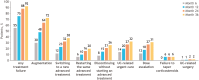
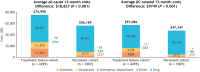
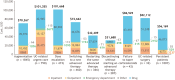
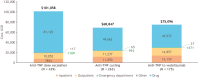
Results: Analysis of treatment failure patterns included data from 6745 patients; HCRU and cost analyses included data from 5302 patients (treatment failure cohort, n = 4295; persistent cohort, n = 1007). In the overall population, 75% experienced treatment failure within the first 12 months (median: 5.1 months). Augmentation with non-advanced therapy (39%) was the most common first treatment failure event. The treatment failure cohort had significantly (P < .001) higher mean costs than the persistent cohort (all-cause, $74 995 vs $56 169; UC-related, $57 096 vs $47 347) mainly attributed to inpatient admissions and outpatient visits. Dose escalation/interval shortening accounted for the highest total costs ($101 668) across treatment failure events.
Conclusions: Advanced therapies for moderate-to-severe UC are associated with high rates of treatment failure and significant economic burden. More efficacious and durable treatments are needed.
Keywords: Ulcerative colitis; biologic therapy; health care costs; outcomes research.
© The Author(s) 2024. Published by Oxford University Press on behalf of Crohn's & Colitis Foundation.
Conflict of interest statement
Komal Gupte-Singh is an employee of Bristol Myers Squibb and holds stock/options in the company. Keith A. Betts, Ella Xiaoyan Du, and Xiaoyu Nie are employees of Analysis Group, Inc., which has received consulting fees from Bristol Myers Squibb. Scott D. Lee has served as a consultant for and/or received research support from Bristol Myers Squibb, Takeda, AbbVie, Janssen, Pfizer, Protagonist, AMT, and TLL Pharmaceutical. Timothy Ritter has nothing to disclose.
Figures

References
-
- Podolsky DK. Yamada’s Textbook of Gastroenterology. 6th ed. Chichester, West Sussex; Hoboken, NJ: John Wiley & Sons Inc.; 2016.
LinkOut - more resources
Full Text Sources
