Effectiveness and safety of telitacicept for refractory generalized myasthenia gravis: a retrospective study
- PMID: 38751755
- PMCID: PMC11095194
- DOI: 10.1177/17562864241251476
Effectiveness and safety of telitacicept for refractory generalized myasthenia gravis: a retrospective study
Abstract
Background: Refractory generalized myasthenia gravis (GMG) remains a substantial therapeutic challenge. Telitacicept, a recombinant human B-lymphocyte stimulator receptor-antibody fusion protein, holds promise for interrupting the immunopathology of this condition.
Objectives: This study retrospectively assessed the effectiveness and safety of telitacicept in patients with refractory GMG.
Design: A single-center retrospective study.
Methods: Patients with refractory GMG receiving telitacicept (160 mg/week or biweekly) from January to September in 2023 were included. We assessed effectiveness using Myasthenia Gravis Foundation of America post-intervention status (MGFA-PIS), myasthenia gravis treatment status and intensity (MGSTI), quantitative myasthenia gravis (QMG), and MG-activity of daily living (ADL) scores, alongside reductions in prednisone dosage at 3- and 6-month intervals. Safety profiles were also evaluated.
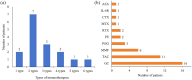
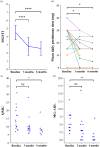
Results: Sixteen patients with MGFA class II-V refractory GMG were included, with eight females and eight males. All patients were followed up for at least 3 months, and 11 patients reached 6 months follow-up. At the 3-month evaluation, 75% (12/16) demonstrated clinical improvement with MGFA-PIS. One patient achieved pharmacological remission, two attained minimal manifestation status, and nine showed functional improvement; three remained unchanged, and one deteriorated. By the 6-month visit, 90.1% (10/11) sustained significant symptomatic improvement. MGSTI scores and prednisone dosages significantly reduced at both follow-ups (p < 0.05). MG-ADL and QMG scores showed marked improvement at 6 months (p < 0.05). The treatment was well tolerated, with no severe adverse events such as allergy or infection reported.
Conclusion: Our exploratory investigation suggests that telitacicept is a feasible and well-tolerated add-on therapy for refractory GMG, offering valuable clinical evidence for this novel treatment option.
Keywords: B lymphocyte; B-cell activating factor; a proliferation-inducing ligand; myasthenia; refractory generalized myasthenia gravis; telitacicept.
© The Author(s), 2024.
Figures

Similar articles
-
Successful treatment of generalized myasthenia gravis with telitacicept: a Chinese case series and literature review.Front Neurol. 2025 Jan 31;16:1501500. doi: 10.3389/fneur.2025.1501500. eCollection 2025. Front Neurol. 2025. PMID: 39958611 Free PMC article.
-
Efgartigimod Followed by Telitacicept in Adult Generalized Myasthenia Gravis: A Retrospective Case Series.J Inflamm Res. 2025 Apr 8;18:4831-4842. doi: 10.2147/JIR.S513986. eCollection 2025. J Inflamm Res. 2025. PMID: 40224392 Free PMC article.
-
A multicenter, randomized, open-label, phase 2 clinical study of telitacicept in adult patients with generalized myasthenia gravis.Eur J Neurol. 2024 Aug;31(8):e16322. doi: 10.1111/ene.16322. Epub 2024 May 10. Eur J Neurol. 2024. PMID: 38726639 Free PMC article. Clinical Trial.
-
Quality of life in refractory generalized myasthenia gravis: A rapid review of the literature.Intractable Rare Dis Res. 2019 Nov;8(4):231-238. doi: 10.5582/irdr.2019.01121. Intractable Rare Dis Res. 2019. PMID: 31890449 Free PMC article. Review.
-
Efficacy and Safety of Immunotherapies in Refractory Myasthenia Gravis: A Systematic Review and Meta-Analysis.Front Neurol. 2021 Dec 1;12:725700. doi: 10.3389/fneur.2021.725700. eCollection 2021. Front Neurol. 2021. PMID: 34925206 Free PMC article.
Cited by
-
Telitacicept in combination with B-cell depletion therapy in MuSK antibody-positive myasthenia gravis: a case report and literature review.Front Immunol. 2024 Nov 18;15:1456822. doi: 10.3389/fimmu.2024.1456822. eCollection 2024. Front Immunol. 2024. PMID: 39624088 Free PMC article. Review.
-
Telitacicept as an alternative to non-steroidal immunosuppressive therapies in the treatment of myasthenia gravis: a study on clinical efficacy and steroid-sparing effect.Front Immunol. 2025 Mar 24;16:1549034. doi: 10.3389/fimmu.2025.1549034. eCollection 2025. Front Immunol. 2025. PMID: 40196119 Free PMC article.
-
Exploring Telitacicept for Neurological Autoimmune Disorders: A Case Study on Morvan Syndrome.Am J Case Rep. 2025 Jun 21;26:e947004. doi: 10.12659/AJCR.947004. Am J Case Rep. 2025. PMID: 40543057 Free PMC article.
-
Successful sequential therapy with rituximab and telitacicept in refractory Anti-NMDA receptor encephalitis and MOG-associated demyelination: a case report and literature review.Front Immunol. 2025 Feb 6;16:1509143. doi: 10.3389/fimmu.2025.1509143. eCollection 2025. Front Immunol. 2025. PMID: 39981240 Free PMC article. Review.
-
Advances in B Cell Targeting for Treating Muscle-Specific Tyrosine Kinase-Associated Myasthenia Gravis.Immunotargets Ther. 2024 Dec 11;13:707-720. doi: 10.2147/ITT.S492062. eCollection 2024. Immunotargets Ther. 2024. PMID: 39678139 Free PMC article. Review.
References
-
- Gilhus NE, Verschuuren JJ. Myasthenia gravis: subgroup classification and therapeutic strategies. Lancet Neurol 2015; 14: 1023–1036. - PubMed
LinkOut - more resources
Full Text Sources

