From Rare Disorders of Kidney Tubules to Acute Renal Injury: Progress and Prospective
- PMID: 38751796
- PMCID: PMC11095595
- DOI: 10.1159/000536423
From Rare Disorders of Kidney Tubules to Acute Renal Injury: Progress and Prospective
Abstract
Background: Acute kidney injury (AKI) is a severe condition marked by rapid renal function deterioration and elevated mortality, with traditional biomarkers lacking sensitivity and specificity. Rare tubulointerstitial diseases encompass a spectrum of disorders, primarily including monogenic diseases, immune-related conditions, and drug-induced tubulointerstitial diseases. The clinical manifestations vary from electrolyte and acid-base imbalances to kidney function insufficiency, which is associated with AKI in up to 20% of cases. Evidence indicated that rare tubulointerstitial diseases might provide new conceptual insights and perspectives for novel biomarkers and potential therapeutic strategies for AKI.
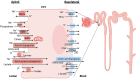
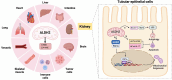
Summary: Autosomal dominant tubulointerstitial kidney disease (ADTKD) and Fanconi syndrome (FS) are rare tubulointerstitial diseases. In ADTKD, UMOD and REN are closely related to AKI by affecting oxidative stress and tubuloglomerular feedback, which provide potential new biomarkers for AKI. Both rare tubulointerstitial diseases and AKI share etiologies and treatment responses. From the mechanism standpoint, rare tubulointerstitial diseases and AKI involve tubular transporter injury, initially manifesting as tubular dysfunction in tubulointerstitial disorder and progressing to AKI because of the programmed cell death with apoptosis, pyroptosis, or necroptosis of proximal tubule cells. Additionally, mitochondrial dysfunction has been identified as a common mechanism in both tubulointerstitial diseases and AKI induced by drugs, pSS, or monoclonal diseases. In the end, both AKI and FS patients and animal models responded well to the therapy of the primary diseases.
Key messages: In this review, we describe an overview of ADTKD and FS to identify their associations with AKI. Mitochondrial dysfunction contributes to rare tubulointerstitial diseases and AKI, which might provide a potential therapeutic target.
Keywords: Acute kidney injury; Mitochondrial disorder; Rare tubulointerstitial diseases.
© 2024 The Author(s). Published by S. Karger AG, Basel.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures

References
-
- Hoste EAJ, Kellum JA, Selby NM, Zarbock A, Palevsky PM, Bagshaw SM, et al. . Global epidemiology and outcomes of acute kidney injury. Nat Rev Nephrol. 2018;14(10):607–25. - PubMed
-
- Patschan D, Erfurt S, Oess S, Lauxmann MA, Patschan S, Ritter O, et al. . Biomarker-based prediction of survival and recovery of kidney function in acute kidney injury. Kidney Blood Press Res. 2023;48(1):124–34. - PubMed
-
- Abuduwupuer Z, Lei Q, Liang S, Xu F, Liang D, Yang X, et al. . The spectrum of biopsy-proven kidney diseases, causes, and renal outcomes in acute kidney injury patients. Nephron. 2023;147(9):541–9. - PubMed
-
- El-Achkar TM, McCracken R, Liu Y, Heitmeier MR, Bourgeois S, Ryerse J, et al. . Tamm-Horsfall protein translocates to the basolateral domain of thick ascending limbs, interstitium, and circulation during recovery from acute kidney injury. Am J Physiol Ren Physiol. 2013;304(8):F1066–75. - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

