IL-6 receptor antibody treatment improves muscle weakness in experimental autoimmune myasthenia gravis mouse model
- PMID: 38751878
- PMCID: PMC11094227
- DOI: 10.3389/fneur.2024.1356300
IL-6 receptor antibody treatment improves muscle weakness in experimental autoimmune myasthenia gravis mouse model
Abstract
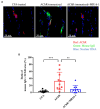
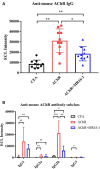
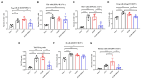
Myasthenia gravis (MG) is a chronic autoimmune disease characterized by muscle weakness and fatigue. It is caused by pathological autoantibodies against components expressed at neuromuscular junctions, such as acetylcholine receptor (AChR). Interleukin-6 (IL-6) has been suggested to play a role in the pathogenesis of MG, and IL-6 receptor (IL-6R) antibody treatment may provide a novel therapeutic option. In this study, we investigated the effects of IL-6R antibody treatment in an experimental autoimmune MG (EAMG) mouse model. We demonstrated that IL-6R antibody treatment improved muscle weakness, reduced IgG deposition at neuromuscular junctions, and the levels of AChR autoantibodies in serum. In addition, follicular helper T cells and Th17, plasma cells in lymph nodes were lower in IL-6R antibody treated mice. Our findings suggest that IL-6R blockade may be a novel and effective therapeutic strategy for the treatment of MG.
Keywords: IL-6; IL-6R antibody; acetylcholine receptor; follicular helper T cells; myasthenia gravis.
Copyright © 2024 Miyake, Serizawa, Onishi, Katsura, Baba, Kurasawa, Tomizawa-Shinohara, Yorozu, Matsumoto and Noguchi-Sasaki.
Conflict of interest statement
SM, KS, SO, YK, MB, MK, HT-S, KY, YM, and MN-S were employed by Chugai Pharmaceutical Co., Ltd.
Figures

Similar articles
-
ATRA alters humoral responses associated with amelioration of EAMG symptoms by balancing Tfh/Tfr helper cell profiles.Clin Immunol. 2013 Aug;148(2):162-76. doi: 10.1016/j.clim.2013.05.009. Epub 2013 May 24. Clin Immunol. 2013. PMID: 23773919
-
Exogenous IL-9 Ameliorates Experimental Autoimmune Myasthenia Gravis Symptoms in Rats.Immunol Invest. 2018 Oct;47(7):712-724. doi: 10.1080/08820139.2018.1487976. Epub 2018 Jun 26. Immunol Invest. 2018. PMID: 29944018
-
Pathological mechanisms in experimental autoimmune myasthenia gravis. II. Passive transfer of experimental autoimmune myasthenia gravis in rats with anti-acetylcholine recepotr antibodies.J Exp Med. 1976 Sep 1;144(3):739-53. doi: 10.1084/jem.144.3.739. J Exp Med. 1976. PMID: 182897 Free PMC article.
-
Novel animal models of acetylcholine receptor antibody-related myasthenia gravis.Ann N Y Acad Sci. 2012 Dec;1274:133-9. doi: 10.1111/j.1749-6632.2012.06773.x. Ann N Y Acad Sci. 2012. PMID: 23252908 Review.
-
Experimental Autoimmune Myasthenia Gravis (EAMG): from immunochemical characterization to therapeutic approaches.J Autoimmun. 2014 Nov;54:51-9. doi: 10.1016/j.jaut.2014.06.003. Epub 2014 Jun 24. J Autoimmun. 2014. PMID: 24970384 Review.
Cited by
-
Povetacicept (ALPN-303; TACI vTD-Fc), an enhanced, potent dual inhibitor of BAFF and APRIL, ameliorates experimental autoimmune myasthenia gravis in C57BL/6N mice.Front Immunol. 2025 Jun 6;16:1533093. doi: 10.3389/fimmu.2025.1533093. eCollection 2025. Front Immunol. 2025. PMID: 40547042 Free PMC article.
References
-
- Howard JF, Jr, Utsugisawa K, Benatar M, Murai H, Barohn RJ, Illa I, et al. . Safety and efficacy of eculizumab in anti-acetylcholine receptor antibody-positive refractory generalised myasthenia gravis (REGAIN): a phase 3, randomised, double-blind, placebo-controlled, multicentre study. Lancet Neurol. (2017) 16:976–86. doi: 10.1016/S1474-4422(17)30369-1, PMID: - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

