Immune checkpoint inhibitors in high-grade upper tract urothelial carcinoma: Paradigm shift emphasizing organ preservation
- PMID: 38751949
- PMCID: PMC11090768
- DOI: 10.1002/bco2.335
Immune checkpoint inhibitors in high-grade upper tract urothelial carcinoma: Paradigm shift emphasizing organ preservation
Erratum in
-
Erratum.BJUI Compass. 2024 Dec 30;5(12):1324-1329. doi: 10.1002/bco2.482. eCollection 2024 Dec. BJUI Compass. 2024. PMID: 39744071 Free PMC article.
Abstract
Objective: The aim was to evaluate the role of immune check point inhibitors (ICIs) in patients with high-grade upper tract urothelial carcinoma (UTUC) who are managed endoscopically when nephroureterectomy (NU) is not feasible, such as in patients who are either not candidates for NU or decline extirpative surgery.
Methods: All patients diagnosed with high-grade UTUC and managed endoscopically between January 1996 and August 2022 were included in the study. Subsequently, patients were categorised based on their use of ICIs into group 1 (patients who did not receive ICIs) and group 2 (patients who received ICIs). Survival outcomes were assessed using Kaplan-Meier analysis, while a multivariable regression model was employed to analyse the impact of clinical characteristics on survival.
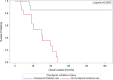
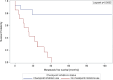
Results: A total of 29 patients were enrolled, with 14 in group 1 and 15 in group 2. Both groups exhibited similar demographic and disease characteristics, including multifocality, laterality and initial tumour size. The median follow-up period was 29.2 months. Notably, group 2 demonstrated significantly enhanced overall and metastasis-free survival rates compared to group 1. At 47.8 months, the overall survival rate was 0% (all patients died) in group 1, whereas it was 85.7% in group 2. Similarly, the metastasis-free survival rate was 0% (all patients had metastatic disease) in group 1 at 40.6 months, whereas it reached 78.0% in group 2. The multivariable analysis indicated a correlation between ICI usage and improved survival outcomes, with a hazard ratio of 0.002.
Conclusion: Utilisation of adjuvant ICIs in the setting of endoscopically treated patients with high-grade UTUC is associated with significantly improved survival rates. ICIs should be considered in this patient population, however, more studies with larger sample size are warranted.
Keywords: high grade; immune checkpoint inhibitors; immunotherapy; renal urothelial carcinoma; upper tract urothelial carcinoma; ureteral carcinoma.
© 2024 The Authors. BJUI Compass published by John Wiley & Sons Ltd on behalf of BJU International Company.
Conflict of interest statement
The authors disclose no potential conflict of interest.
Figures
References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials