Modeling airway persistent infection of Moraxella catarrhalis and nontypeable Haemophilus influenzae by using human in vitro models
- PMID: 38751999
- PMCID: PMC11094313
- DOI: 10.3389/fcimb.2024.1397940
Modeling airway persistent infection of Moraxella catarrhalis and nontypeable Haemophilus influenzae by using human in vitro models
Abstract
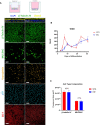
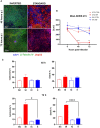
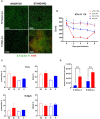
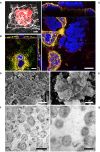
Non-typeable Haemophilus influenzae (NTHi) and Moraxella catarrhalis (Mcat) are two common respiratory tract pathogens often associated with acute exacerbations in Chronic Obstructive Pulmonary Disease (COPD) as well as with otitis media (OM) in children. Although there is evidence that these pathogens can adopt persistence mechanisms such as biofilm formation, the precise means through which they contribute to disease severity and chronicity remains incompletely understood, posing challenges for their effective eradication. The identification of potential vaccine candidates frequently entails the characterization of the host-pathogen interplay in vitro even though this approach is limited by the fact that conventional models do not permit long term bacterial infections. In the present work, by using air-liquid-interface (ALI) human airway in vitro models, we aimed to recreate COPD-related persistent bacterial infections. In particular, we explored an alternative use of the ALI system consisting in the assembly of an inverted epithelium grown on the basal part of a transwell membrane with the aim to enable the functionality of natural defense mechanisms such as mucociliary clearance and cellular extrusion that are usually hampered during conventional ALI infection experiments. The inversion of the epithelium did not affect tissue differentiation and considerably delayed NTHi or Mcat infection progression, allowing one to monitor host-pathogen interactions for up to three weeks. Notably, the use of these models, coupled with confocal and transmission electron microscopy, revealed unique features associated with NTHi and Mcat infection, highlighting persistence strategies including the formation of intracellular bacterial communities (IBCs) and surface-associated biofilm-like structures. Overall, this study demonstrates the possibility to perform long term host-pathogen investigations in vitro with the aim to define persistence mechanisms adopted by respiratory pathogens and individuate potential new vaccine targets.
Keywords: AECOPD; CIVM; airways; in vitro models; microscopy; replacement.
Copyright © 2024 Ariolli, Canè, Di Fede, Tavarini, Taddei, Buno, Delany, Rossi Paccani and Pezzicoli.
Conflict of interest statement
At the time of the study AA was recipient of a GSK fellowship from the PhD program of the University of Tuscia. MC was a PhD student recipient of a PhD fellowship funded by the MIUR at the University of Naples Federico II. AT is an employee of the University of Tuscia. MD, ST, KB, ID, SP and AP are employees of the GSK group of companies. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Host-pathogen interaction profiling of nontypeable Haemophilus influenzae and Moraxella catarrhalis coinfection of bronchial epithelial cells.mSphere. 2025 Jul 29;10(7):e0024225. doi: 10.1128/msphere.00242-25. Epub 2025 Jun 10. mSphere. 2025. PMID: 40492732 Free PMC article.
-
Indirect pathogenicity of Haemophilus influenzae and Moraxella catarrhalis in polymicrobial otitis media occurs via interspecies quorum signaling.mBio. 2010 Jul 6;1(3):e00102-10. doi: 10.1128/mBio.00102-10. mBio. 2010. PMID: 20802829 Free PMC article.
-
Antibodies against the Majority Subunit (PilA) of the Type IV Pilus of Nontypeable Haemophilus influenzae Disperse Moraxella catarrhalis from a Dual-Species Biofilm.mBio. 2018 Dec 11;9(6):e02423-18. doi: 10.1128/mBio.02423-18. mBio. 2018. PMID: 30538189 Free PMC article.
-
Insights on persistent airway infection by non-typeable Haemophilus influenzae in chronic obstructive pulmonary disease.Pathog Dis. 2017 Jun 1;75(4):ftx042. doi: 10.1093/femspd/ftx042. Pathog Dis. 2017. PMID: 28449098 Free PMC article. Review.
-
The Role of Non-Typeable Haemophilus influenzae Biofilms in Chronic Obstructive Pulmonary Disease.Front Cell Infect Microbiol. 2021 Aug 4;11:720742. doi: 10.3389/fcimb.2021.720742. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 34422683 Free PMC article. Review.
References
MeSH terms
LinkOut - more resources
Full Text Sources

