Efficacy, immunogenicity, and safety of COVID-19 vaccines in individuals with liver cirrhosis: a rapid review and meta-analysis
- PMID: 38752003
- PMCID: PMC11091435
- DOI: 10.7774/cevr.2024.13.2.83
Efficacy, immunogenicity, and safety of COVID-19 vaccines in individuals with liver cirrhosis: a rapid review and meta-analysis
Abstract
The emergence of coronavirus disease 2019 (COVID-19) vaccines has been a remarkable advancement. However, the efficacy, immunogenicity, and safety of these vaccines in individuals with liver cirrhosis require careful evaluation due to their compromised immune status and potential interactions with underlying liver disease. The present study aimed to evaluate the safety and efficacy of COVID-19 vaccines in liver cirrhosis patients. In the present study, we searched international databases, including Google Scholar, PubMed, Scopus, Embase, and Web of Science. The search strategy was carried out by using keywords and MeSH (Medical Subject Headings) terms. STATA ver. 15.0 (Stata Corp., USA) was used to analyze the data statistically. The analysis was performed using the random-effects model. We also used the chi-square test and I2 index to calculate heterogeneity among studies. For evaluating publication bias, Begg's funnel plots and Egger's tests were used. A total of 4,831 liver cirrhosis patients with COVID-19 were examined from 11 studies. The rate of hospitalization in the patients with liver cirrhosis was 17.6% (95% confidence interval [CI], 9%-44%). The rate of fever in the patients with liver cirrhosis was 4.5% (95% CI, 0.9%-8.1%). The rate of positive neutralizing antibodies in the patients with liver cirrhosis was 82.5% (95% CI, 69.8%-95.1%). Also, the rates of seroconversion after the second vaccination in patients with liver cirrhosis and the control group were 96.6% (95% CI, 92.0%-99.0%), and 99.7% (95% CI, 99.0%-100.0%), respectively. COVID-19 vaccines have demonstrated promising efficacy, immunogenicity, and safety profiles in individuals with liver cirrhosis, providing crucial protection against COVID-19-related complications.
Keywords: COVID-19 vaccine; Efficacy; Immunogenicity; Liver cirrhosis; Safety.
© Korean Vaccine Society.
Conflict of interest statement
No potential conflict of interest relevant to this article was reported.
Figures
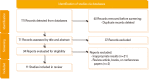
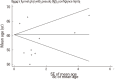

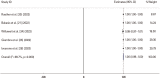
Similar articles
-
Efficacy, immunogenicity and safety of COVID-19 vaccines in older adults: a systematic review and meta-analysis.Front Immunol. 2022 Sep 13;13:965971. doi: 10.3389/fimmu.2022.965971. eCollection 2022. Front Immunol. 2022. PMID: 36177017 Free PMC article.
-
Immunogenicity of COVID-19 vaccines in patients with cirrhosis: A meta-analysis.Hum Vaccin Immunother. 2024 Dec 31;20(1):2326316. doi: 10.1080/21645515.2024.2326316. Epub 2024 Mar 11. Hum Vaccin Immunother. 2024. PMID: 38466197 Free PMC article.
-
A randomized, double-blind, placebo-controlled phase III clinical trial to evaluate the efficacy and safety of SARS-CoV-2 vaccine (inactivated, Vero cell): a structured summary of a study protocol for a randomised controlled trial.Trials. 2021 Apr 13;22(1):276. doi: 10.1186/s13063-021-05180-1. Trials. 2021. PMID: 33849629 Free PMC article.
-
COVID-19 vaccine immunogenicity among chronic liver disease patients and liver transplant recipients: A meta-analysis.Clin Mol Hepatol. 2022 Oct;28(4):890-911. doi: 10.3350/cmh.2022.0087. Epub 2022 Jun 3. Clin Mol Hepatol. 2022. PMID: 36263669 Free PMC article.
-
Efficacy, Immunogenicity and Safety of COVID-19 Vaccines: A Systematic Review and Meta-Analysis.Front Immunol. 2021 Oct 11;12:714170. doi: 10.3389/fimmu.2021.714170. eCollection 2021. Front Immunol. 2021. PMID: 34707602 Free PMC article.
References
-
- Shafieipour S, Rezaei Zadeh Rukerd M, Shamsizadeh Meymandi T, et al. The effect of intravenous tocilizumab therapy on the prognosis of patients with COVID-19: a case-control study. Iran J Med Microbiol. 2023;17:243–250.
-
- Bottcher K, Pinzani M. Pathophysiology of liver fibrosis and the methodological barriers to the development of anti-fibrogenic agents. Adv Drug Deliv Rev. 2017;121:3–8. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

