Safety assessments of recombinant DTaP vaccines developed in South Korea
- PMID: 38752005
- PMCID: PMC11091433
- DOI: 10.7774/cevr.2024.13.2.155
Safety assessments of recombinant DTaP vaccines developed in South Korea
Abstract
Purpose: Pertussis bacteria have many pathogenic and virulent antigens and severe adverse reactions have occurred when using inactivated whole-cell pertussis vaccines. Therefore, inactivated acellular pertussis (aP) vaccines and genetically detoxified recombinant pertussis (rP) vaccines are being developed. The aim of this study was to assess the safety profile of a novel rP vaccine under development in comparison to commercial diphtheria-tetanus-acellular pertussis (DTaP) vaccines.
Materials and methods: The two positive control DTaP vaccines (two- and tri-components aP vaccines) and two experimental recombinant DTaP (rDTaP) vaccine (two- and tri-components aP vaccines adsorbed to either aluminum hydroxide or purified oat beta-glucan) were used. Temperature histamine sensitization test (HIST), indirect Chinese hamster ovary (CHO) cell cluster assay, mouse-weight-gain (MWG) test, leukocytosis promoting (LP) test, and intramuscular inflammatory cytokine assay of the injection site performed for safety assessments.
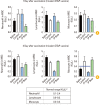
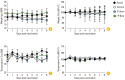
Results: HIST results showed absence of residual pertussis toxin (PTx) in both control and experimental DTaP vaccine groups, whereas in groups immunized with tri-components vaccines, the experimental tri-components rDTaP absorbed to alum showed an ultra-small amount of 0.0066 IU/mL. CHO cell clustering was observed from 4 IU/mL in all groups. LP tests showed that neutrophils and lymphocytes were in the normal range in all groups immunized with the two components vaccine. However, in the tri-components control DTaP vaccine group, as well as two- and tri-components rDTaP with beta-glucan group, a higher monocyte count was observed 3 days after vaccination, although less than 2 times the normal range. In the MWG test, both groups showed changes less than 20% in body temperature and body weight before the after the final immunizations. Inflammatory cytokines within the muscle at the injection site on day 3 after intramuscular injection revealed no significant response in all groups.
Conclusion: There were no findings associated with residual PTx, and no significant differences in both local and systemic adverse reactions in the novel rDTaP vaccine compared to existing available DTaP vaccines. The results suggest that the novel rDTaP vaccine is safe.
Keywords: Bordetella pertussis; Diphtheria-tetanus-acellular pertussis vaccines; Murine model study; Recombinant DTaP vaccine; Safety.
© Korean Vaccine Society.
Conflict of interest statement
No potential conflict of interest relevant to this article was reported.
Figures




Similar articles
-
Comparative Evaluation of Recombinant and Acellular Pertussis Vaccines in a Murine Model.Vaccines (Basel). 2024 Jan 22;12(1):108. doi: 10.3390/vaccines12010108. Vaccines (Basel). 2024. PMID: 38276680 Free PMC article.
-
A safety and immunogenicity comparison of 12 acellular pertussis vaccines and one whole-cell pertussis vaccine given as a fourth dose in 15- to 20-month-old children.Pediatrics. 1997 Nov;100(5):772-88. doi: 10.1542/peds.100.5.772. Pediatrics. 1997. PMID: 9346976 Clinical Trial.
-
Effect of priming with diphtheria and tetanus toxoids combined with whole-cell pertussis vaccine or with acellular pertussis vaccine on the safety and immunogenicity of a booster dose of an acellular pertussis vaccine containing a genetically inactivated pertussis toxin in fifteen- to twenty-one-month-old children. Italian Multicenter Group for the Study of Recombinant Acellular Pertussis Vaccine.J Pediatr. 1995 Aug;127(2):238-43. doi: 10.1016/s0022-3476(95)70301-2. J Pediatr. 1995. PMID: 7636648 Clinical Trial.
-
Diphtheria-tetanus-acellular pertussis vaccine adsorbed (Triacelluvax; DTaP3-CB): a review of its use in the prevention of Bordetella pertussis infection.Paediatr Drugs. 2000 Mar-Apr;2(2):139-59. doi: 10.2165/00148581-200002020-00007. Paediatr Drugs. 2000. PMID: 10937466 Review.
-
A cellular pertussis vaccine (Infanrix-DTPa; SB-3). A review of its immunogenicity, protective efficacy and tolerability in the prevention of Bordetella pertussis infection.Drugs. 1996 Aug;52(2):254-75. doi: 10.2165/00003495-199652020-00010. Drugs. 1996. PMID: 8841742 Review.
References
-
- Dorji D, Mooi F, Yantorno O, Deora R, Graham RM, Mukkur TK. Bordetella pertussis virulence factors in the continuing evolution of whooping cough vaccines for improved performance. Med Microbiol Immunol. 2018;207:3–26. - PubMed
-
- Corbel MJ, Xing DK. Toxicity and potency evaluation of pertussis vaccines. Expert Rev Vaccines. 2004;3:89–101. - PubMed
-
- Higgs R, Higgins SC, Ross PJ, Mills KH. Immunity to the respiratory pathogen Bordetella pertussis. Mucosal Immunol. 2012;5:485–500. - PubMed
-
- Gustafsson L, Hallander HO, Olin P, Reizenstein E, Storsaeter J. A controlled trial of a two-component acellular, a five-component acellular, and a whole-cell pertussis vaccine. N Engl J Med. 1996;334:349–355. - PubMed
LinkOut - more resources
Full Text Sources

